US Health Agency Endorses Bio-Thera's Avzivi® as Alternative to Avastin®, Authorizing Bevacizumab-tnjn Use
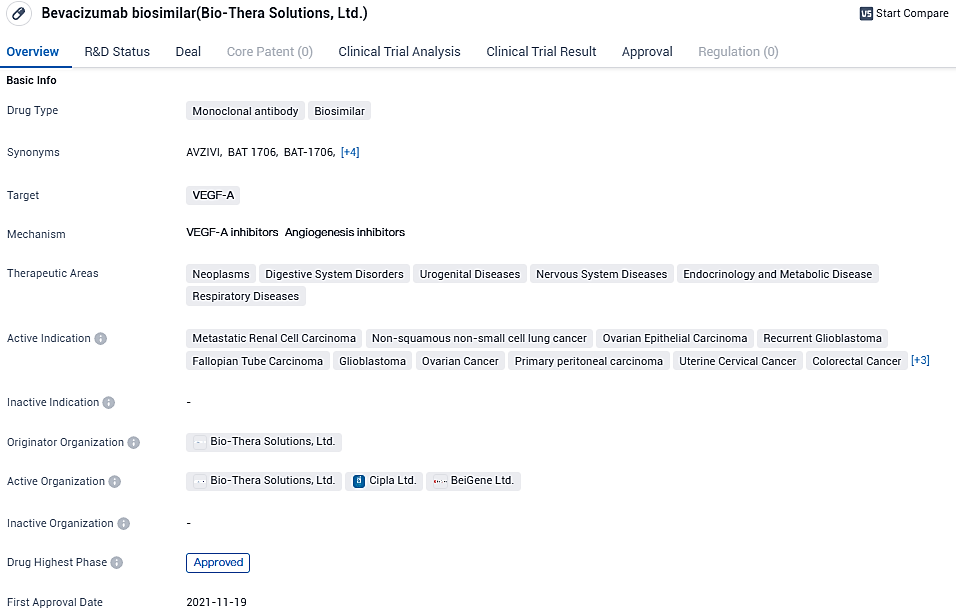
Bio-Thera Solutions, Ltd, an enterprise actively engaged in the biopharmaceutical field with a focus on bringing to market both biosimilar products and novel therapies, has made public the approval of their product Avzivi® (bevacizumab-tnjn) by the United States Food and Drug Administration. Avzivi® is a biosimilar that corresponds to the reference product Avastin®.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Avzivi®, Bio-Thera's novel biosimilar, has achieved the milestone of being the second among its products to secure authorization from the United States Food and Drug Administration (USFDA). This makes it one of the pioneering biosimilars to be conceived, crafted, and launched by a Chinese drug maker to obtain the FDA's nod within the United States.
The sanction from the FDA for Avzivi® arose from a detailed package of analytical, preclinical, and clinical evidence submitted by Bio-Thera. The comparison of BAT1706 with Avastin® from both the US and EU markets involved rigorous analytical examinations. These delved into the structural, physicochemical, and biological attributes to affirm the biosimilarity of BAT1706.
Clinical assessments were performed through a controlled, blinded phase III study using parallel groups. Comparing BAT1706 to the reference drug Avastin® involved evaluating the efficacy, safety, and immunogenic response in patients facing advanced forms of non-squamous non-small cell lung cancer. The accumulated data verified that BAT1706 matched the reference biologic in terms of efficacy, safety, immunogenic potential, and overall quality.
Bio-Thera's CEO, Dr. Shengfeng Li, expressed elation over the approval of Avzivi®, noting it as a significant stride for the company. This marks the second authorization from the FDA for Bio-Thera in the US market. Dr. Li emphasized the company's dedication to advancing their biosimilar portfolio and the goal to acquire further biosimilar approvals, thereby enhancing the availability of critical medical treatments to patients.
In September 2021, Bio-Thera and Sandoz formed a strategic alliance through a licensing and commercialization deal for Avzivi® (BAT1706). According to this partnership, Bio-Thera assumes the responsibility for the advancement and production of the biosimilar. Meanwhile, Sandoz will take charge of the marketing and distribution of Avzivi® within the US and additional international markets.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
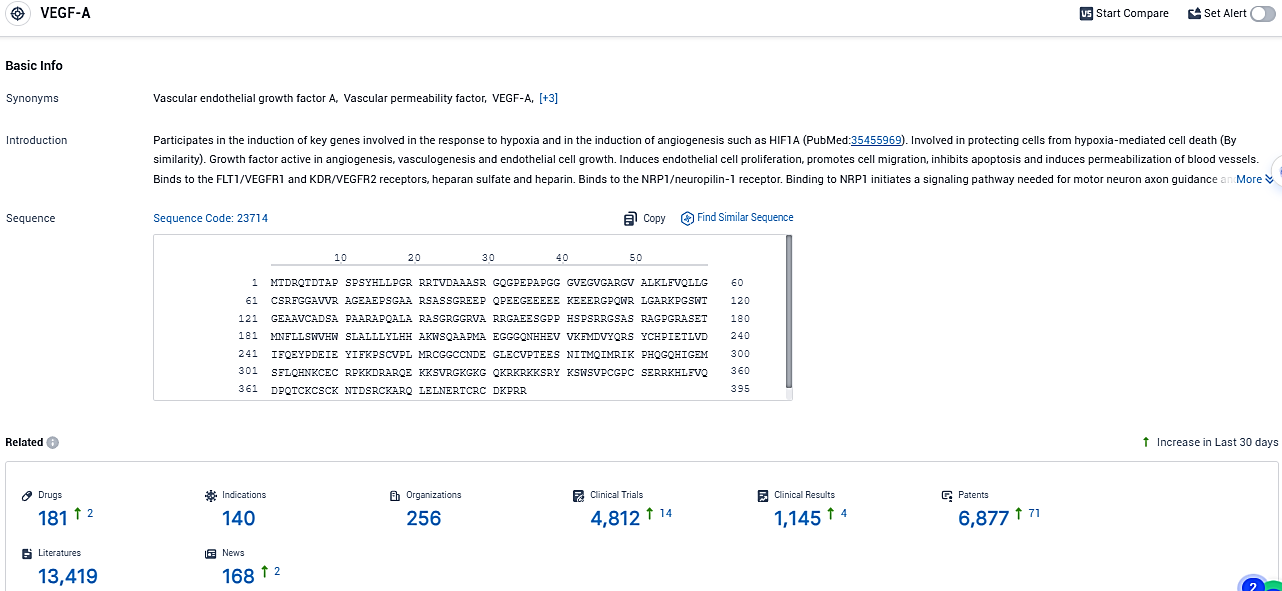
According to the data provided by the Synapse Database, As of December 15, 2023, there are 181 investigational drugs for the VEGF-A target, including 140 indications, 256 R&D institutions involved, with related clinical trials reaching 4812, and as many as 6877 patents.
Avzivi® (bevacizumab-tnjn) is a humanized monoclonal antibody that targets VEGF. Bevacizumab has shown promise in treating various therapeutic areas, particularly in the field of oncology. With its wide range of indications and recent approval in China, the drug holds potential for improving the treatment options available for patients with different types of cancers.