Alvotech and STADA expand their strategic alliance with a denosumab collaboration
Alvotech and STADA are enhancing their current strategic collaboration focused on producing high-quality, affordable biosimilars. They are expanding their joint efforts to include AVT03, a biosimilar candidate in clinical development that uses Prolia/Xgeva (denosumab) as reference drugs aimed at treating osteoporosis and cancer-induced bone deterioration.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
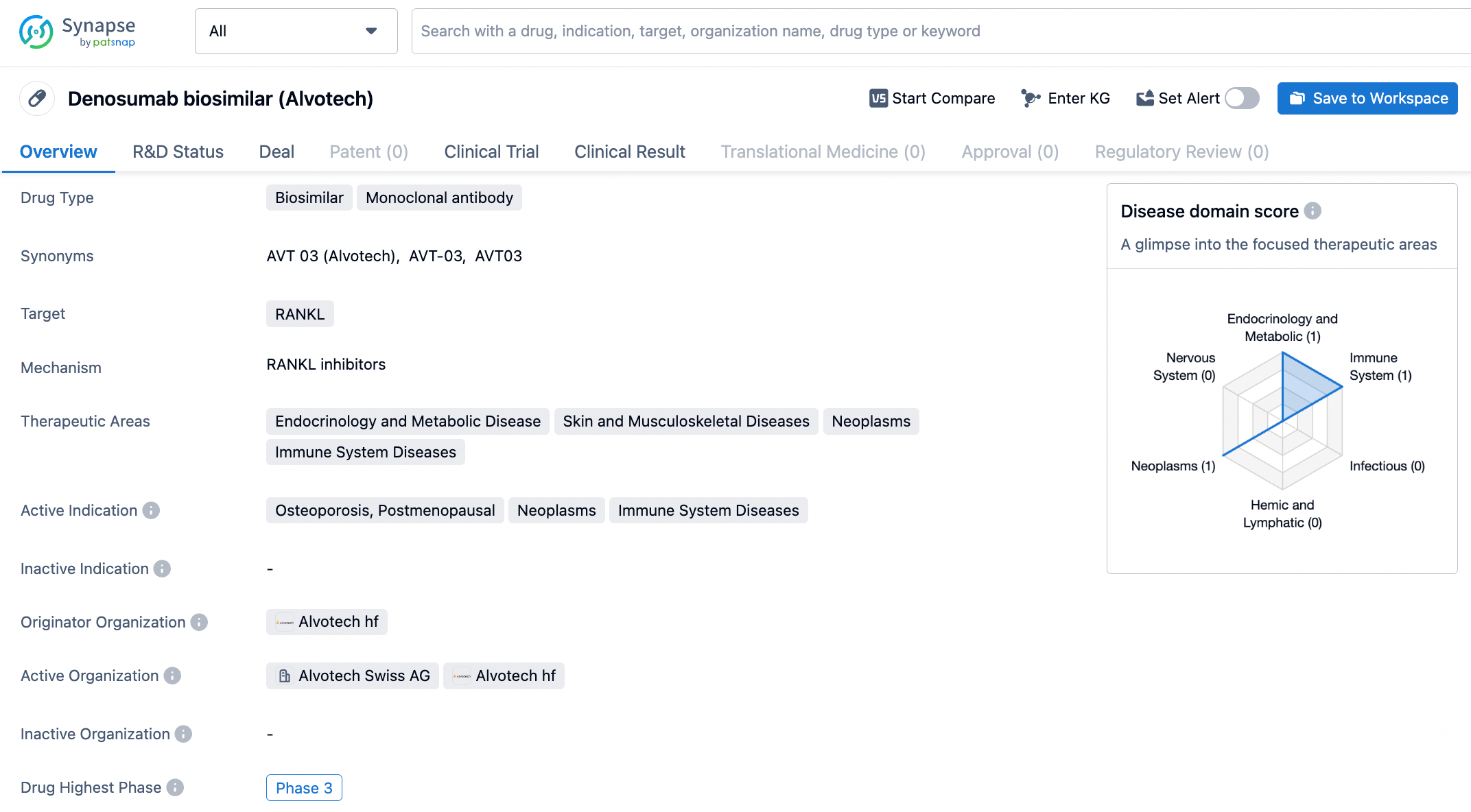
According to the agreement, Alvotech will oversee development and production at its advanced facility located in Reykjavik, Iceland. Upon receiving approval for AVT03, STADA will acquire marketing authorization and take on semi-exclusive commercial rights in Europe, including Switzerland and the UK, as well as exclusive commercial rights in select countries within Central Asia and the Middle East.
Alongside the AVT03 commercial agreement, the partners have decided to expand STADA's commercial rights to biosimilars for Humira (adalimumab) and Stelara® (ustekinumab) within the Commonwealth of Independent States in Central Asia. Additionally, Alvotech will reclaim commercial rights for AVT06, a planned biosimilar to Eylea (aflibercept), from STADA.
Bryan Kim, STADA’s Global Specialty Head, stated: “As the European market leader with our teriparatide osteoporosis therapy, Movymia, STADA recognizes significant potential to provide patients and clinicians with another treatment option, denosumab. Our strong oncology presence, evidenced by our six currently marketed biosimilars, underscores the value of expanding our partnership with Alvotech to optimize the use of our resources."
Anil Okay, Alvotech's Chief Commercial Officer, added: “We anticipate continued collaboration with STADA to enhance patient access to affordable biologics in the denosumab market, similar to our success with our citrate-free, high-concentration Humira biosimilar. This expansion of our commercial partnership confirms Alvotech’s dedicated focus on biosimilar development and production, as well as our robust comprehensive capabilities and expertise.”
Denosumab is a human monoclonal IgG2 antibody that targets the RANKL protein, which is vital for the development, function, and survival of osteoclasts, the cells responsible for bone resorption. The increased osteoclast activity stimulated by RANKL is a major factor in bone destruction associated with metastatic bone disease.
Denosumab binds to RANKL with high specificity and affinity, preventing the interaction between RANKL and RANK. This results in a decrease in osteoclast numbers and activity, leading to reduced bone resorption and bone destruction induced by cancer.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
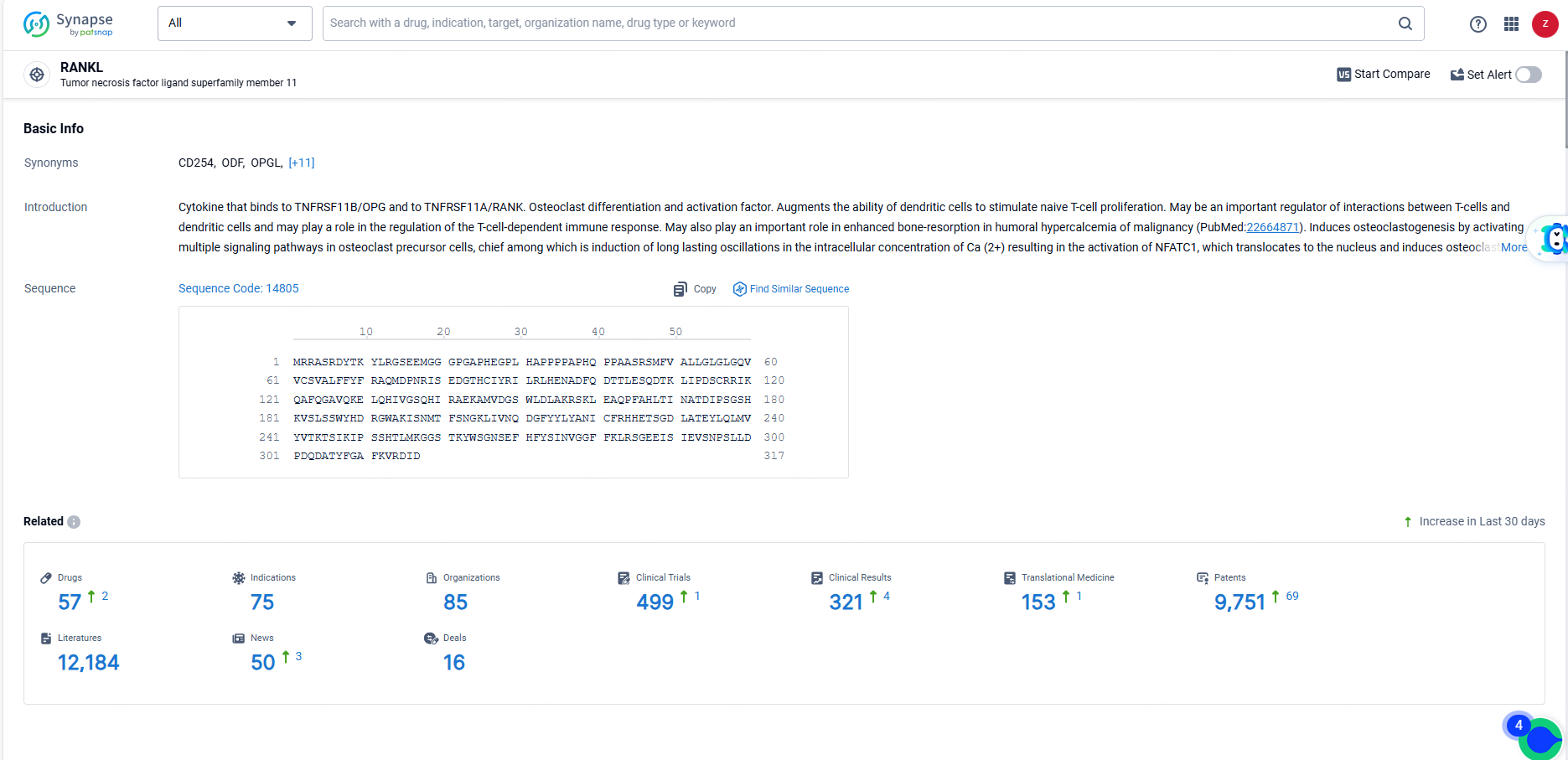
According to the data provided by the Synapse Database, As of June 14, 2024, there are 57 investigational drugs for the RANKL targets, including 75 indications, 85 R&D institutions involved, with related clinical trials reaching 499, and as many as 9751 patents.
AVT03 is a biosimilar candidate for Prolia and Xgeva (denosumab), medicines for osteoporosis and bone cancer, respectively. Denosumab is a human monoclonal IgG2 antibody that targets the protein RANKL, which is essential for the formation, function and survival of osteoclasts, the cell type responsible for bone resorption. AVT03 is an investigational product and has not received regulatory approval in any country.