Alvotech Signs Exclusive License Contract with Kashiv BioSciences for Marketing of Xolair®
Alvotech, a worldwide biotechnology firm known for the creation and production of biosimilar drugs, along with Kashiv Biosciences LLC, a comprehensive biopharmaceutical enterprise, have publicized their exclusive licensing accord for AVT23 (also called ADL018). This proposed biosimilar to Xolair® (omalizumab) is currently undergoing clinical trials.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The contract pertains to all 27 nations of the European Union, alongside the UK, Australia, Canada, and New Zealand. Alvotech will gain a unique right to market AVT23, which is undergoing design and production by Kashiv, according to the agreement's specifics. An initial payment from Kashiv is expected, with potential for follow-up payments based on progress and obtainable royalty.
"We are thrilled to formalize this exclusive development and marketing agreement for omalizumab. It's an indication of how Alvotech can utilize its platform to tap into promising markets, either through internal product development and manufacturing or in-licensing. In this case, we have the opportunity to implement Alvotech’s expertise in market accessibility as well as capitalize on our worldwide commercial associations," said Alvotech's Chairman and CEO, Robert Wessman.
"We are incredibly excited about granting commercial rights of our omalizumab biosimilar program to Alvotech, a multinational biosimilar firm, for numerous crucial territories. This exclusive development and marketing agreement paves the way for additional patient enrollment in our global Phase III clinical trial, aiming to broaden the reach of this therapy for patients," stated Kashiv's CEO, Dr. Sandeep Gupta.
Omalizumab is a humanized monoclonal antibody that targets free IgE. The drug Xolair, consisting of omalizumab, is recommended for disorders such as extreme persistent allergic asthma, long-term rhinosinusitis with nasal polyps, and chronic spontaneous urticaria. Global sales of Xolair in the year preceding June 30, 2023, approximated at around $3.7 billion.
AVT23, proposed as a biosimilar to Xolair (omalizumab), is an experimental compound that has yet to be approved by any regulatory authority. Until recognized by regulatory authorities, biosimilarity remains unestablished and unclaimed.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
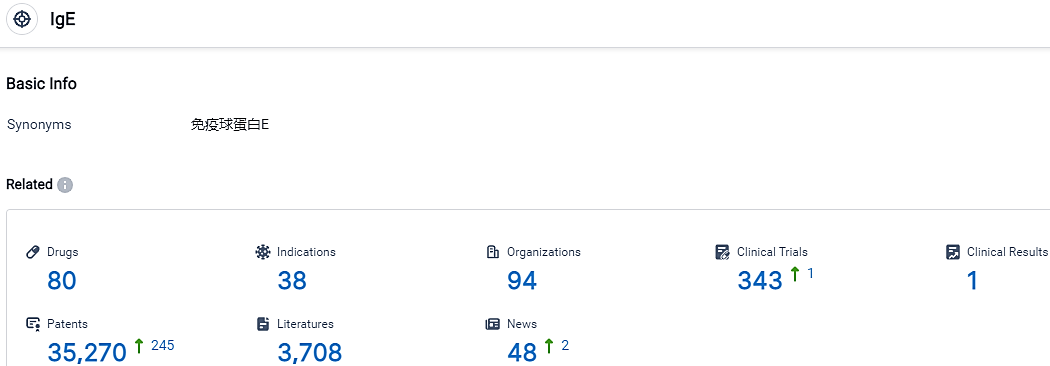
According to the data provided by the Synapse Database, As of October 11, 2023, there are 80 investigational drugs for the IgE target, including 38 indications, 94 R&D institutions involved, with related clinical trials reaching 343,and as many as 35270 patents.
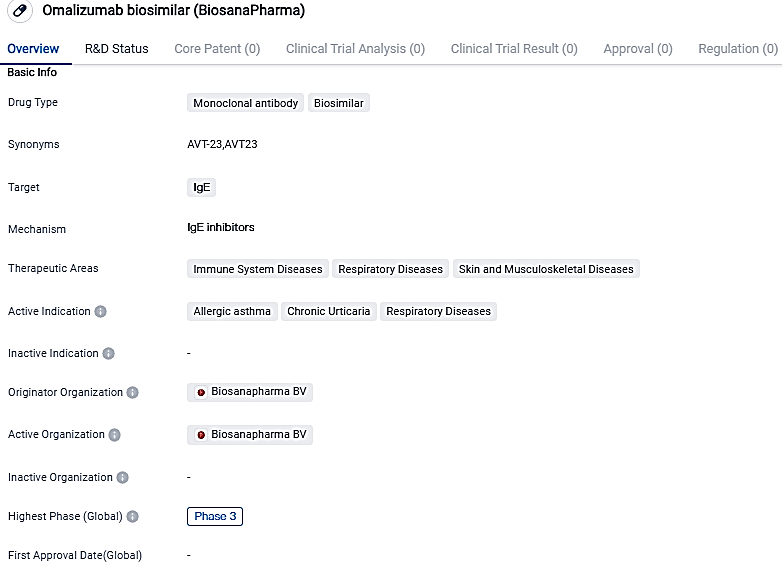
Omalizumab biosimilar is a monoclonal antibody drug targeting IgE. It is intended for the treatment of immune system diseases, respiratory diseases, and skin and musculoskeletal diseases. With active indications including allergic asthma, chronic urticaria, and respiratory diseases, Omalizumab biosimilar holds promise in addressing these conditions.