ALX Oncology Announces Preliminary Results from Stage 2 Clinical Trial of Evorpacept for HER2-Positive Stomach Cancer
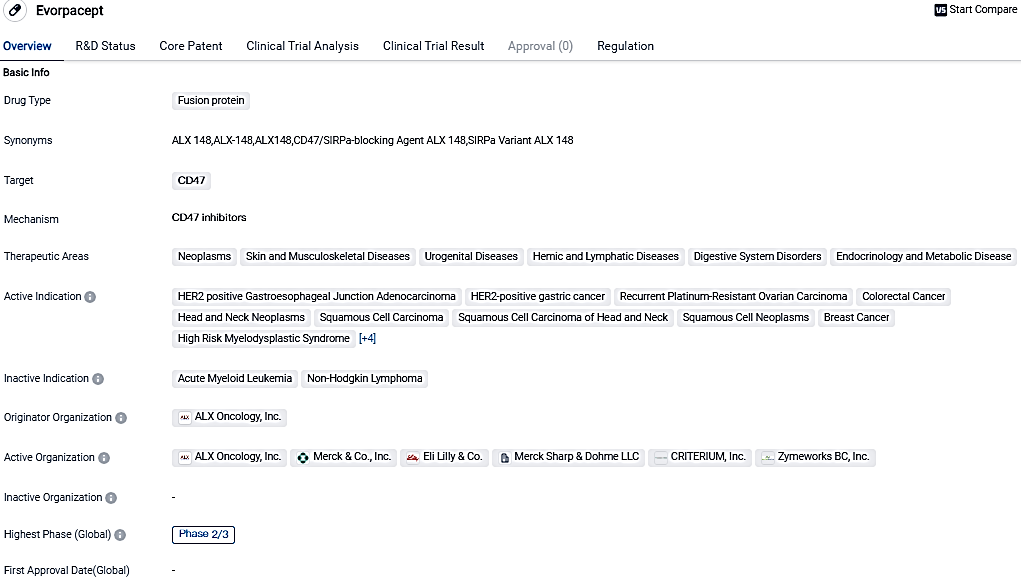
ALX Oncology Holdings Inc., a firm specializing in immuno-oncology and developing treatments targeting the CD47 immune checkpoint pathway, has revealed optimistic preliminary findings from the Phase 2 section of its ASPEN-06 clinical trial. This randomized global multi-center study is evaluating the combination of evorpacept, the firm's CD47 blocker, with trastuzumab, CYRAMZA®, and paclitaxel for helping patients diagnosed with HER2-positive gastric/gastroesophageal junction cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The set interim analysis report shows the outcomes from 54 patients randomly chosen who were dealing with second and third phase gastric/GEJ cancer, and an important amount of these patients were earlier treated with ENHERTU® and checkpoint inhibitors. The patients received evorpacept treatment at the dosage of 30 mg/kg in a bi-weekly manner, which aligns with the treatment pattern of CYRAMZA, trastuzumab, and paclitaxel.
“The ASPEN-06 clinical examination verifies the potential of evorpacept both in solid tumors and when used together with anti-cancer antibodies and this information underscores the potential of this drug as a groundbreaking immunotherapy," conveyed Sophia Randolph, M.D., Ph.D., CMO, at ALX Oncology.
“We're highly motivated by the primary randomized effectiveness and safety outcomes in gastric cancer which enhance the activity seen beforehand in our initial human trial and serves as the first positive randomized clinical trial reported for any CD47 inhibitor. We anticipate to disclose the final analysis of the ongoing phase 2 ASPEN-06 study in 2024's second quarter and plan to activate the Phase 3 section of ASPEN-06 towards the end of 2024," added Sophia Randolph.
“These results in gastric cancer are the inaugural positive initial outcomes in a randomized trial scheme of obstructing the CD47 immune checkpoint pathway with a CD47 inhibitor possessing an inactive Fc effector capacity," expressed Keun Wook Lee, M.D., Ph.D., who is a Professor at Seoul National University College of Medicine. “Evorpacept has the potential to bring about a transformation in treatment and possibly shift the paradigm in the patient care cycle for gastric cancer."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
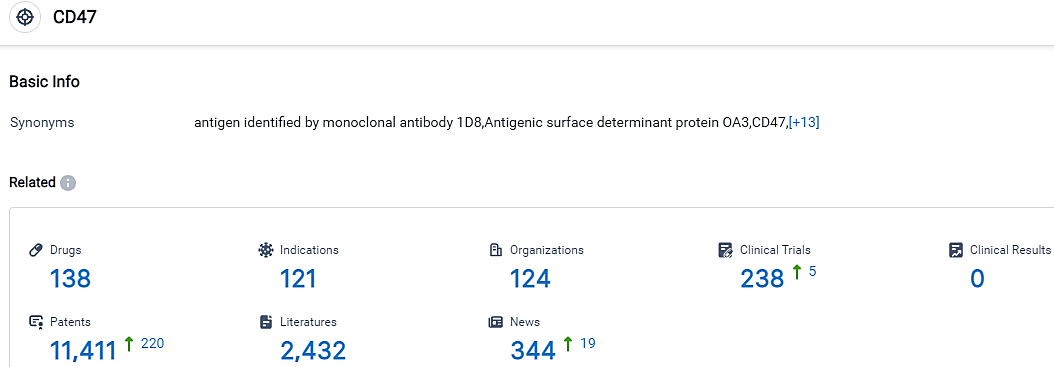
According to the data provided by the Synapse Database, As of October 11, 2023, there are 138 investigational drugs for the CD47 target, including 121 indications, 124 R&D institutions involved, with related clinical trials reaching 238,and as many as 11411 patents.
Evorpacept appears to be a promising drug in the field of biomedicine. Its fusion protein nature and targeting of CD47 make it a potentially effective treatment for various diseases, particularly different types of cancer. The drug's current phase of development and regulatory designations further highlight its potential and the interest it has garnered in the pharmaceutical industry.