Syndax publicizes AUGMENT-101 Study reached its main objective involving Revumenib in KMT2Ar Acute Leukemia cases
Syndax Pharmaceuticals, a biopharmaceutical firm engaged in the clinical phase and developing cutting-edge cancer treatments, has reported encouraging preliminary figures following a protocol-defined collective examination of the essential AUGMENT-101 study on revumenib, a front-runner in the class of menin inhibitors. The trial involved adults and youngsters with cases of recurring or stubborn KMT2A rearranged acute myeloid and lymphoid leukemia.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As recommended by the Independent Data Monitoring Committee, the trial will be halted for additional accrual in the KMT2Ar groups by the Company. Syndax remains confident about submitting a NDA for revumenib, for the R/R KMT2Ar acute leukemia treatment, to the U.S. FDA by the end of the current year.
"We are extremely satisfied to announce encouraging results for revumenib in KMT2Ar acute leukemia cases. These results signal the clinical significance and underscore the prospective of revumenib as a pioneering and top-tier resolution," expressed Michael A. Metzger, Syndax's Chief Executive Officer.
Michael A. Metzger also noted, "Our efforts to submit a New Drug Application by the close of the year have been facilitated by Breakthrough Therapy Designation, which has allowed us to collaborate closely with the FDA. Syndax's financial health is assured till the latter half of 2025, inclusive of various significant milestones and inaugural product releases in 2024, positioning us to tap into the extensive potential of both revumenib and axatilimab."
In the pivotal trial of the KMT2Ar groups, AUGMENT-101 has enlisted a sum of 94 acute leukemia patients until the July 2023 data threshold. From these, 57 had their KMT2Ar status centrally confirmed and were viable for efficacy evaluation following appropriate follow-up. The predominance of the patients evaluated for efficacy had relapsed subsequent to at least one rescue treatment prior to their enrollment, with nearly half of them having undertaken previous stem cell transplant.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
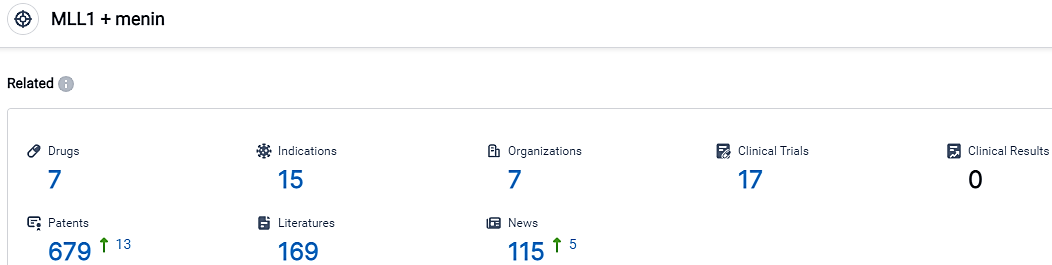
According to the data provided by the Synapse Database, As of October 11, 2023, there are 7 investigational drugs for the MLL1 and menin target, including 15 indications, 7 R&D institutions involved, with related clinical trials reaching 17,and as many as 679 patents.
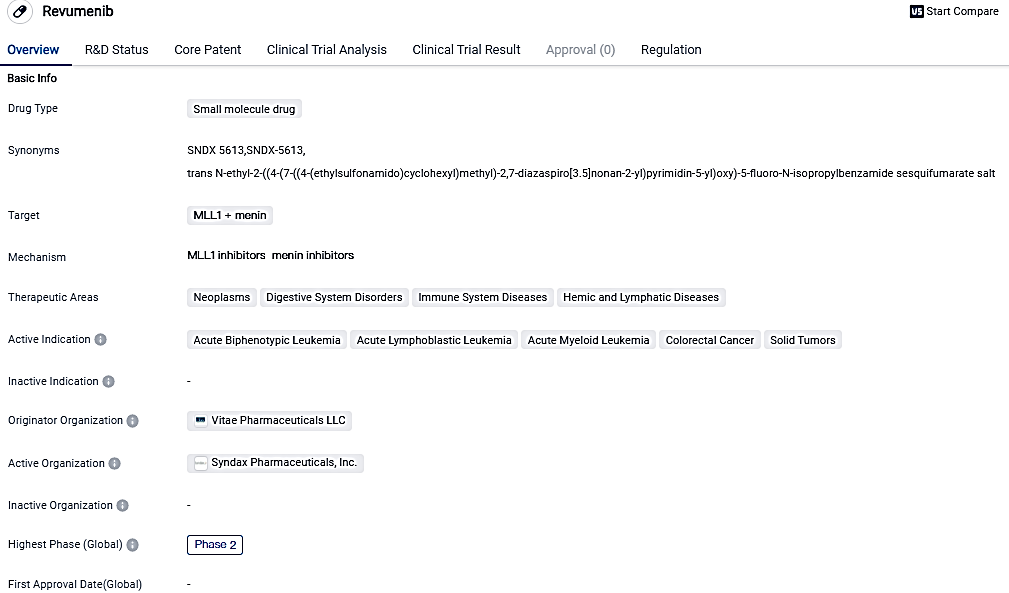
Revumenib is a small molecule drug that targets MLL1 and menin. It has shown potential in treating various therapeutic areas, including neoplasms, digestive system disorders, immune system diseases, and hemic and lymphatic diseases. The drug has received regulatory designations such as Fast Track, Orphan Drug, and Breakthrough Therapy. Its targeting of specific proteins involved in cancer development suggests its potential as a novel treatment approach.