ALX Oncology Highlights Promising Anticancer Effects of Evorpacept and Zanidatamab in Advanced Breast Cancer
ALX Oncology Holdings Inc. (Nasdaq: ALXO) is a biotechnology firm in the clinical development stage, focused on therapies that enhance the immune system for cancer treatment and improving patient survival rates. The company reported outcomes from a Phase 1b/2 clinical trial, which shows that their experimental CD47-blocker, evorpacept, in combination with Jazz Pharmaceuticals’ zanidatamab, exhibits promising anti-tumor effects in patients with advanced breast cancer that is HER2-positive or HER2-low. This is the first report from a clinical trial assessing the safety and efficacy of evorpacept alongside zanidatamab in patients with metastatic breast cancer (mBC) who have undergone extensive prior treatments. These results will be showcased during a poster spotlight presentation (#PS8-09) at the 2024 San Antonio Breast Cancer Symposium (SABCS) on Thursday, December 12.
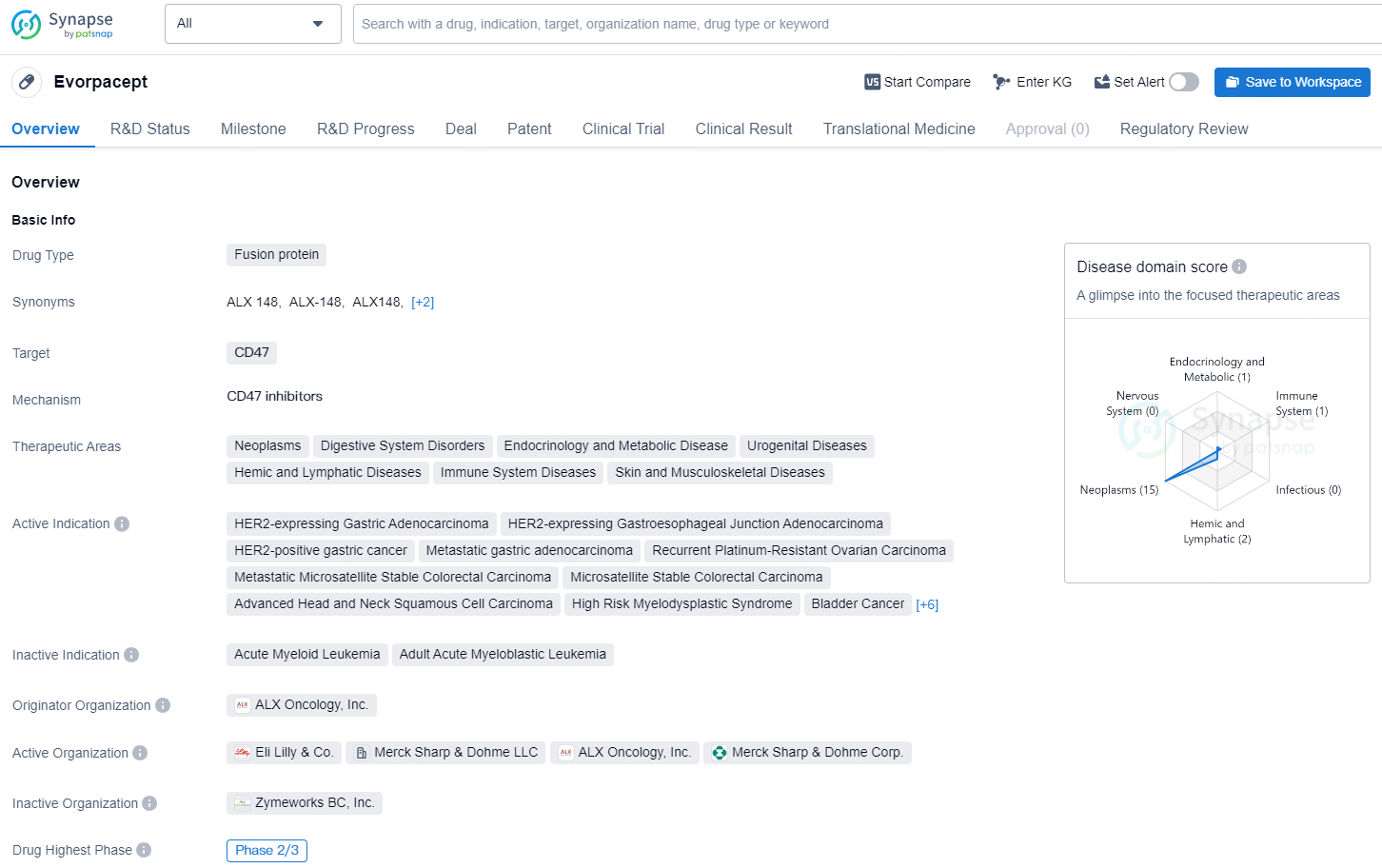
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Dr. Alberto J. Montero, M.D., MBA, Clinical Director of the Breast Cancer Medical Oncology Program at Case Western Reserve University and principal investigator of the study, stated, “The data indicate that patients with HER2-positive cancer who have undergone extensive prior treatment may gain from CD47 inhibition through the unique action of evorpacept when it is paired with a HER2-targeted treatment. There is an urgent need for new treatment options with improved safety for these patients, especially once their disease advances after receiving traditional therapies like ENHERTU.”
The Phase 1b/2 open-label, multi-center clinical trial (NCT05027139) investigated the efficacy of evorpacept, a novel CD47 blocker currently under investigation, in conjunction with zanidatamab, a bispecific antibody targeting HER2, for patients with previously treated inoperable, locally advanced, or metastatic HER2-expressing breast cancer and other malignancies.
The first part of the study focused on determining the safety and optimal dosage for the drug combination, while the second part evaluated its anti-tumor efficacy. The poster presentation at SABCS will report on the effectiveness of all three cohorts from the second part of the trial: Cohort 1 (n=21) included patients with HER2-positive breast cancer, who had undergone a median of six systemic therapies in the metastatic setting, and all had prior treatment with fam-trastuzumab deruxtecan-nxki (ENHERTU®). Patients were enrolled based either on local tumor sample evaluations or central assessments, with nine out of the 21 patients in Cohort 1 confirmed as HER2-positive via central evaluation. Cohort 2 (n=15) comprised individuals with HER2-low breast cancer, who had a median of five prior systemic therapies, while Cohort 3 (n=8) included patients with other HER2-expressing cancers.
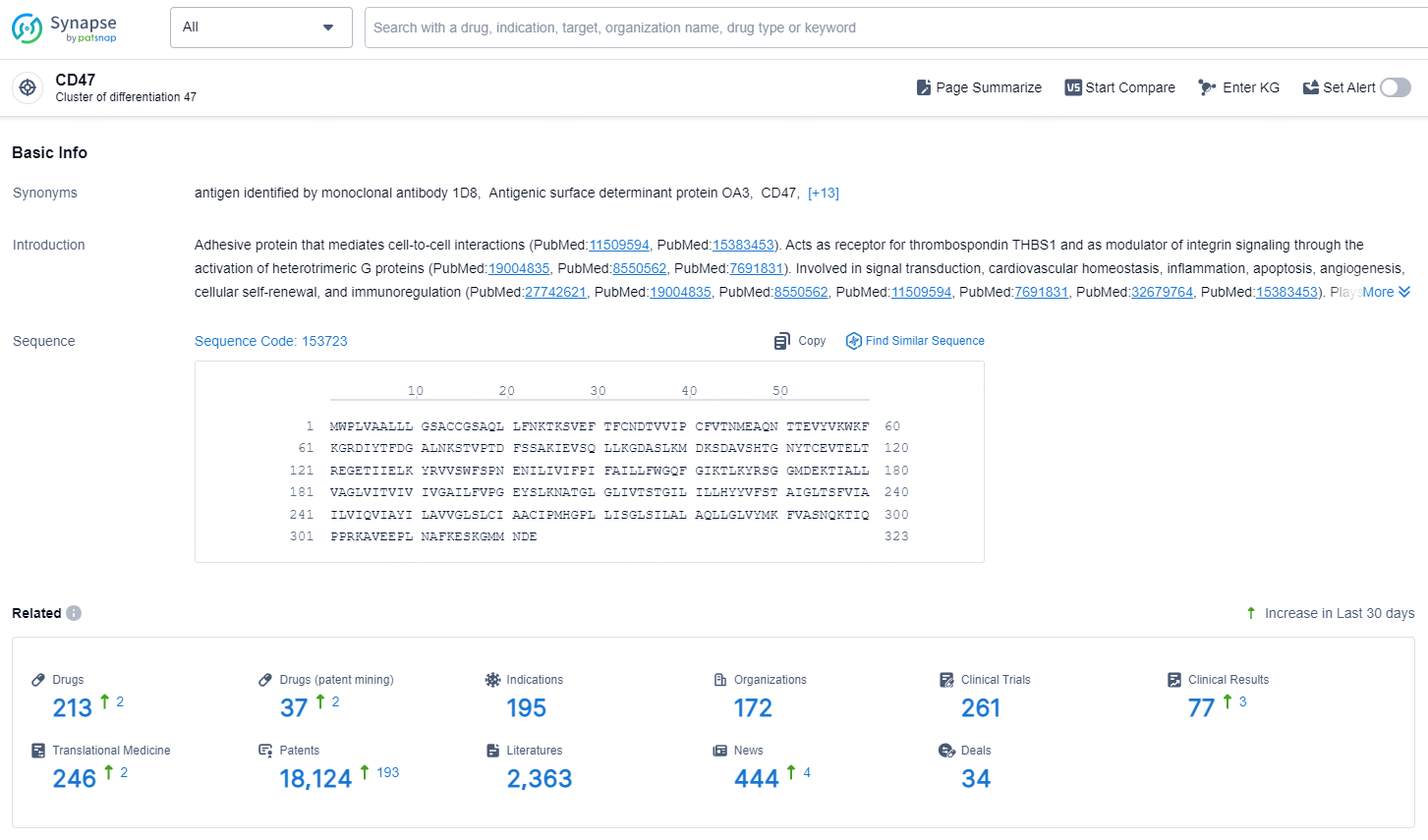
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 17, 2024, there are 213 investigational drugs for the CD47 target, including 195 indications, 172 R&D institutions involved, with related clinical trials reaching 261, and as many as 18124 patents.
Evorpacept is a fusion protein drug developed by ALX Oncology, Inc. It is currently in the highest global phase of development at Phase 2/3. The drug targets CD47 and is being developed for the treatment of various therapeutic areas, including neoplasms, digestive system disorders, endocrinology and metabolic diseases, urogenital diseases, hemic and lymphatic diseases, immune system diseases, and skin and musculoskeletal diseases.