Alzheon Announces Clinical Advances Therapy in Phase 2 Study of ALZ-801 Administered Orally to Early Alzheimer's Patients
Alzheon, Inc., which is involved in the clinical-stage biopharmaceutical sphere with a focus on creating a range of product candidates and diagnostic tests for individuals affected by Alzheimer's disease and comparable neurodegenerative ailments, reported a measurably significant decrease in blood biomarkers of neural degradation. In addition, they observed maintenance of cerebral capacity and encouraging cognitive effects among initial AD patients who have the apolipoprotein ε4 allele subsequent to a two-year treatment using the experimental compound ALZ-801 in the Phase 2 biomarker study.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"We are encouraged by the increasing amount of data that backs the possibility of ALZ-801 becoming the inaugural oral disease-altering therapy for Alzheimer's. The extensive reduction of the plasma p-tau181 biomarker when contrasted with plaque-clearing anti-amyloid antibodies, bolsters our belief that ALZ-801 can reshape Alzheimer's treatment methods," said Martin Tolar, MD, PhD, Founder, President, and CEO of Alzheon.
ALZ-801 is currently being examined as an oral disease-altering treatment in Phase 3 development for Early AD management. During action mechanism studies, ALZ-801 completely halted the production of neurotoxic soluble beta-amyloid oligomers at the Phase 3 clinical dosage. Oral ALZ-801 has shown promising signs of powerful clinical efficiency in the high-risk and extremely vulnerable late-onset Alzheimer’s population - patients carrying double copies of the apolipoprotein ε4 allele.
In the single-arm, multicentre, open-labelled Phase 2 biomarker trial, the biomarker effects, clinical benefits and safety measures of the ALZ-801 tablet were assessed in 84 Early AD patients. These patients possess either one or two copies of the ε4 allele of the apolipoprotein E gene and indicated presence of amyloid and tau biomarkers in their cerebrospinal fluid. The APOE4 genotype, the most significant risk factor for AD besides ageing, is linked with high levels of neurotoxic amyloid oligomers in the brain.
Phosphorylated tau (p-tau) elevation in plasma serves as a reliable and sensitive marker of neuronal stress and brain impairment in AD and is viewed as highly trustworthy core biomarker of disease pathology. P-tau is generated by neurons in response to the creation of neurotoxic beta amyloid oligomers, which are primarily responsible for AD pathology and neurodegeneration. P-tau181 concentrations increase with the onset of AD symptoms and with the clinical worsening of patients. It's also been seen to decrease when administered clinically effective anti-amyloid disease-altering treatments for Alzheimer’s.
"Alzheon's work in developing an oral precision medicine targeting neurotoxic amyloid oligomers in Alzheimer’s disease is truly groundbreaking. The imaging and clinical data regarding ALZ-801 strengthens this approach," said Kaj Blennow, MD, PhD, Professor of Clinical Neurochemistry at the University of Gothenburg, Sweden. "In several trials of anti-amyloid treatments, p-tau181 has emerged as a constant plasma biomarker that appears to correlate with clinical benefits and backs the expectation of treatment’s clinical effectiveness.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
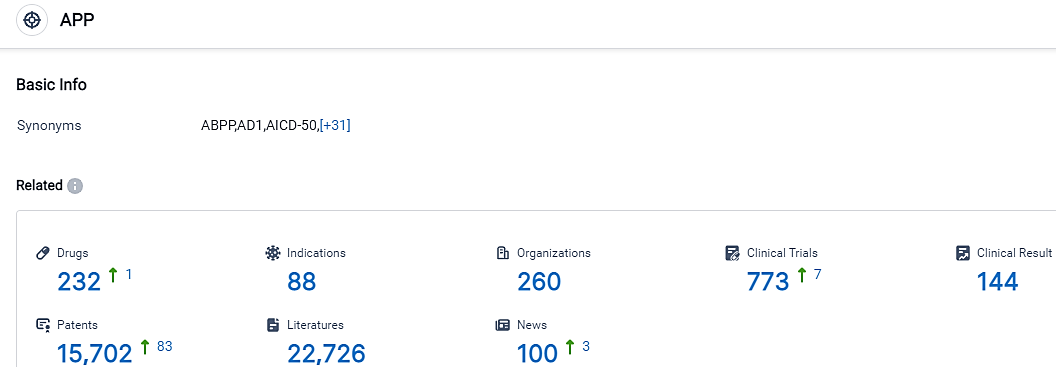
According to the data provided by the Synapse Database, As of September 18, 2023, there are 232 investigational drugs for the APP target, including 88 indications, 260 R&D institutions involved, with related clinical trials reaching 773,and as many as 15702 patents.
ALZ-801 is an investigational oral agent in Phase 3 development as a potentially disease modifying treatment for AD. It blocks the formation of neurotoxic soluble beta amyloid oligomers causing cognitive decline in Alzheimer’s patients. ALZ-801 received Fast Track designation from the U.S. Food and Drug Administration in 2017.