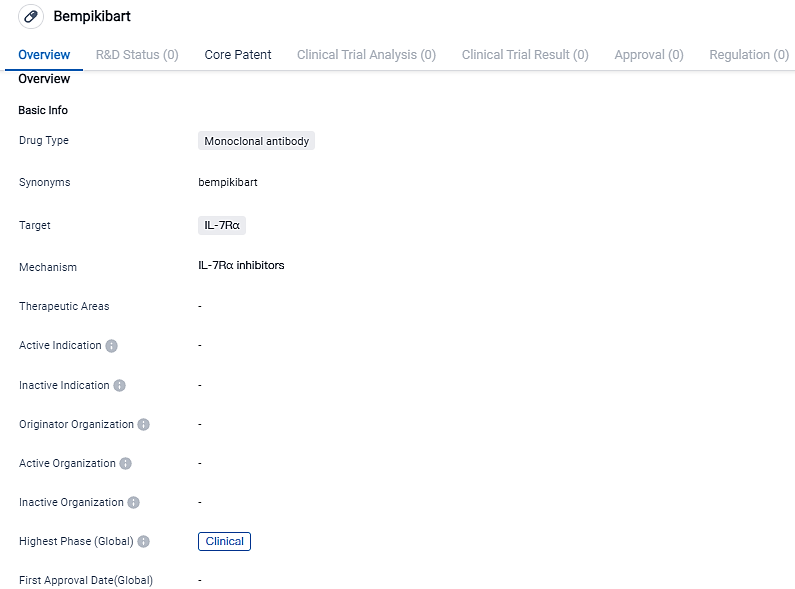
The initiation patient treatment in Phase 2 clinical trials of Bempikibart for Alopecia Areata has been announced by Q32 Bio and Horizon Therapeutics plc
IQ32 Bio, a firm in its clinical phase devoted to the creation of biologic treatment methods that reinstate immune balance, alongside Horizon Therapeutics plc, confirmed the initiation of their Phase 2 multicenter, double-blind, placebo-controlled, proof-of-concept research by giving the first dosage to a patient. The trial aims to measure the efficiency of bempikibart in severe alopecia areata adult sufferers.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Q32 Bio is teaming up with Horizon to work on the creation of bempikibart, a completely human anti-IL-7Rα antibody that aids in balancing the adaptive immune function by thwarting the signaling driven by both IL-7 and TSLP, which are two vital immune pathways.
"Administering the first treatment to a patient in our Phase 2 Alopecia Areata trial and our continuing Phase 2 study for Atopic Dermatitis are indicative of the wide range of potential uses for bempikibart and our steadfast commitment to enhance the lives of individuals suffering from autoimmune and inflammatory ailments," noted Jodie Morrison, a Q32 Bio board member and interim CEO. She noted that Q32 Bio and Horizon share a commitment to proficiently moving bempikibart through both Phase 2 trials.
According to Horizon's Executive Vice President of Research and Development, Elizabeth H.Z. Thompson, Ph.D., "Alopecia areata is a multifaceted ailment with a monumental unmet medical demand, where patients may suffer from patchy to complete hair loss, affecting their lifestyle." She observes that, "The unique potential of bempikibart lies in its ability to rectify immunological imbalance in this and other similar conditions."
Alopecia areata is an autoimmune condition that affects hair follicles leading to acute hair loss. It is non-scarring and can show up at any time. It is the second-highest cause of hair loss and is linked with co-occurring conditions like depression, anxiety, and other autoimmune diseases such as lupus erythematosus and vitiligo. It is randomly prevalent but more commonly seen in women and people of African American descent in the U.S. Worldwide, an estimated 2% of people suffer from this condition.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
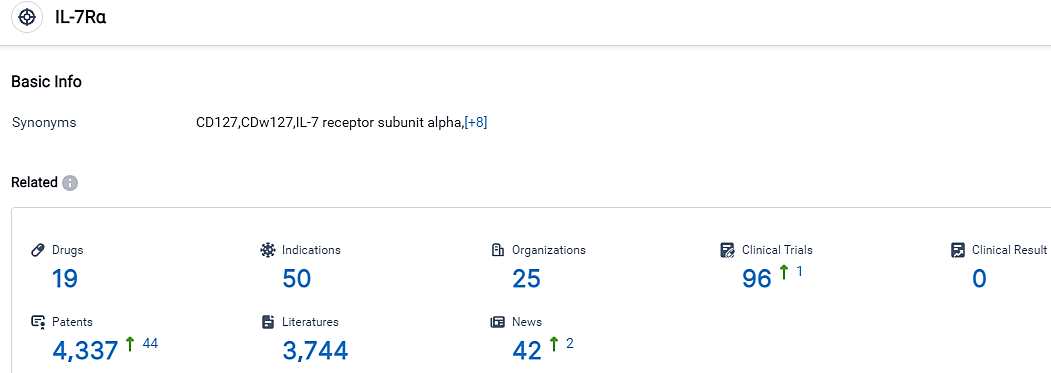
According to the data provided by the Synapse Database, As of September 18, 2023, there are 19 investigational drugs for the IL-7Rα target, including 50 indications, 25 R&D institutions involved, with related clinical trials reaching 96,and as many as 4337 patents.
Bempikibart is a fully human anti-IL-7Rα antibody that re-regulates adaptive immune function by blocking signaling mediated by both IL-7 and TSLP. As a Business Development expert, my role would be to assess the market potential and identify opportunities for Bempikibart in the pharmaceutical industry.