AM-Pharma Begins Stage 2 Clinical Study on Ilofotase Alfa for Renal Injury Linked to Heart Surgery Procedures
AM-Pharma B.V. has declared the commencement of patient treatments within a Phase 2 clinical trial. This study is critical as it assesses the efficacy of the company's unique enzyme, ilofotase alfa, in reducing the risk of kidney injury following cardiac surgery. Worldwide, more than two million individuals undergo open heart surgeries annually, and renal damage connected to such procedures—known as CSA-RD—poses a significant health risk without a dedicated therapeutic drug available.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
CSA-RD is characterized by a progressive decline in kidney function that occurs over time in individuals who have undergone heart surgery, typically stemming from an initial incidence of acute kidney injury that develops shortly after the operation. About 33% of patients who have heart surgery experience CSA-AKI, and it's a leading contributor to mortality following the procedure. Indeed, between 2 to 5 percent of these patients will need kidney replacement treatment following their surgery.
Ilofotase alfa is known for its acceptable safety profile and has shown promising evidence of kidney protection in past clinical research. Impressively, early-stage and advanced-stage clinical trials involving ilofotase alfa have shown notable enhancement in the rate of Major Adverse Kidney Events within 90 days in patients with AKI linked to sepsis. Based on a recent study from the REVIVAL Phase 3 trial, patients treated with ilofotase alfa had a reduction in MAKE90 occurrences, with 57.2% experiencing these adverse events compared to the 64.7% seen in the placebo cohort.
“Clinical studies have indicated that ilofotase alfa has considerable renal protective benefits for those suffering from sepsis-induced AKI. Considering AKI's impact on lasting kidney damage among heart surgery patients, prophylactic use of ilofotase alfa could result in significant improvement in kidney health and even have the potential to reduce mortality,” commented Peter Pickkers, MD, PhD, a recognized authority in Experimental Intensive Care Medicine at Radboud University Nijmegen Medical Centre.
The upcoming multicenter Phase 2 study, which adopts a double-blind, randomized, and placebo-controlled design, aims to investigate the efficacy and safety of ilofotase alfa when used preemptively in 150 cardiac surgery patients who are at risk of developing CSA-RD. The key measure for success will be the comparison of creatinine levels measured before and after the surgical procedure. This trial will also look at other critical outcomes, such as the occurrence of Major Adverse Kidney Events and the incidence and severity of postoperative AKI. Completion of the trial is targeted for the fourth quarter of 2024, with findings anticipated in the early months of 2025.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
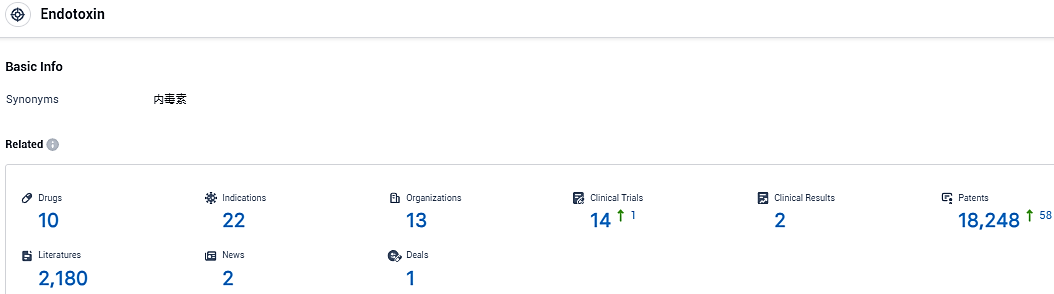
According to the data provided by the Synapse Database, As of January 19, 2024, there are 10 investigational drugs for the endotoxin target, including 22 indications, 13 R&D institutions involved, with related clinical trials reaching 14, and as many as 18248 patents.
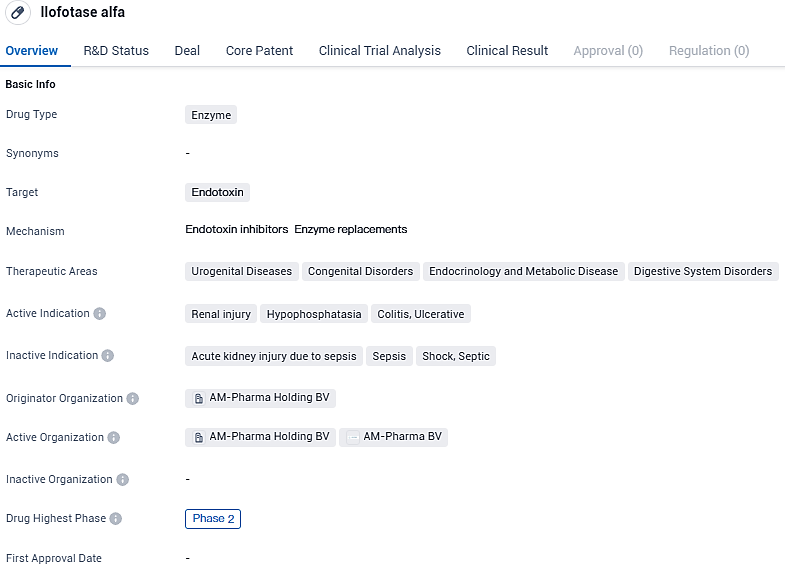
Ilofotase alfa targets endotoxin and is being investigated for its potential in treating urogenital diseases, congenital disorders, endocrinology and metabolic diseases, as well as digestive system disorders. The drug is currently in Phase 2 of clinical trials and has shown promise in addressing conditions such as renal injury, hypophosphatasia, colitis, and ulcerative conditions. Further research is needed to determine its safety and efficacy in larger patient populations.