Encouraging Results in Initial Australian Trial for Androgenic Hair Loss Treatment
Hope Medicine Inc., a pioneering firm engaged in the development of new biopharmaceutical treatments, has made a public declaration confirming the successful conclusion of its Phase Ib clinical trial. The trial, known as "An Open-Label Study for Assessing Safety, Tolerability, and Effectiveness in Male and Female Subjects Affected by Androgenetic Alopecia Using HMI-115 Across a 24-Week Treatment Span," marks a significant milestone for the company's research endeavors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Under the direction of Professor Rodney Sinclair, a research initiative has been conducted involving 16 individuals in Australia, specifically encompassing 12 men and 4 women who suffer from androgenic alopecia. The compound HMI-115 has shown to be both efficacious and safe based on the outcomes of the study and has been well received by the participants. Notably, an average increase of 14 non-vellus hairs per square centimeter was observed among the male participants at the conclusion of the study, a result which was statistically meaningful, signaling a clear benefit from the treatment.
Commenting on the findings, Professor Rui-Ping Xiao, the innovator behind Hope Medicine, stated: "The data from this study brings new hope, marking the first instance where blocking the prolactin receptor has been evidenced to spur hair regrowth in those plagued by androgenic alopecia. It potentially carves out a new direction for therapeutic intervention."
Nathan Chen, driving force and chief executive at Hope Medicine, expressed positivity regarding the initial study, noting: "Buoyed by these preliminary results, we've proceeded to initiate Phase II of our study within China, targeting a pool of 180 patients with androgenic alopecia. By the culmination of 2024, our goal is to have a comprehensive Proof of Concept in place."
Androgenetic Alopecia remains the most prevalent form of hair loss, impacting an estimated 70% of the male populace and 40% of females. It is generally accepted that the condition arises from a mix of genetic factors and the influence of the male hormone dihydrotestosterone, although the precise workings are yet to be completely deciphered. Our investigations suggest a pivotal role for the signaling pathways involving prolactin and its receptors in the development of this disorder.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
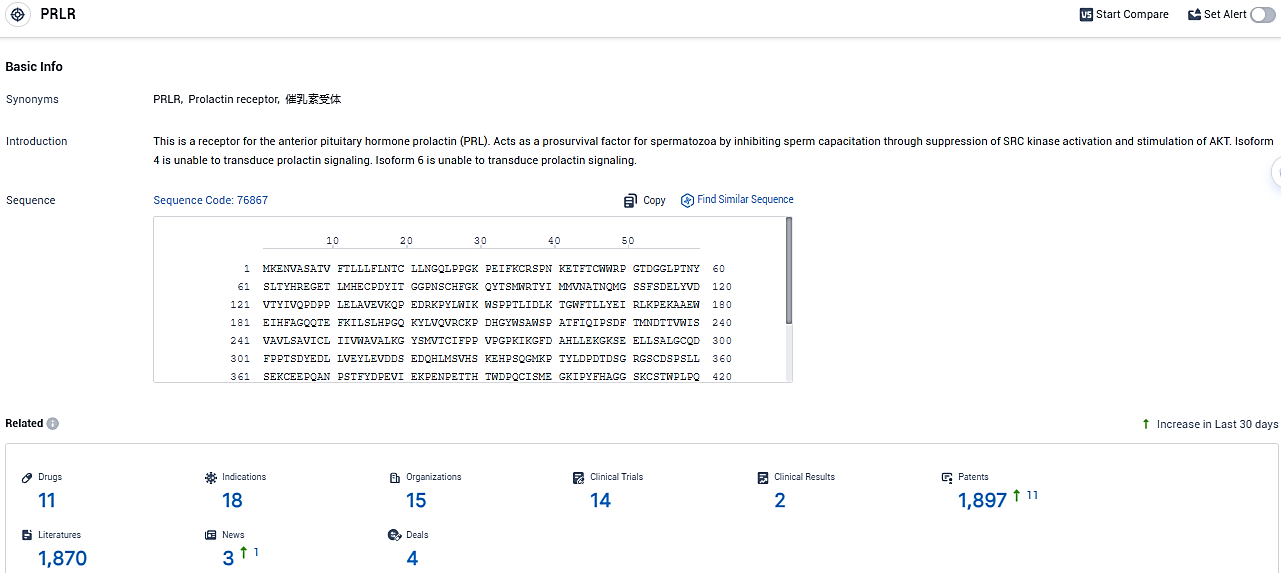
According to the data provided by the Synapse Database, As of January 16, 2024, there are 11 investigational drugs for the PRLR target, including 18 indications, 15 R&D institutions involved, with related clinical trials reaching 14, and as many as 1897 patents.
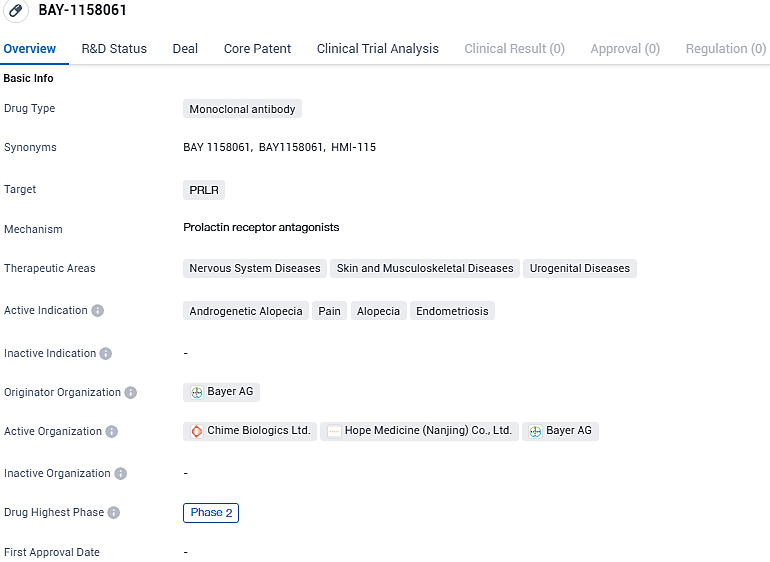
HMI-115 is a monoclonal antibody drug targeting the PRLR and being developed for the treatment of various diseases in the nervous system, skin and musculoskeletal system, and urogenital system. With active indications including androgenetic alopecia, pain, alopecia, and endometriosis, the drug shows promise in addressing a range of medical conditions.