ExeVir Bio Announces Positive Virus Neutralization Data for XVR012 Against Emerging COVID-19 Omicron Variants
ExeVir Bio, a biotech company developing robust heavy chain-only antibody therapies for broad protection against infectious diseases, announced new data demonstrating that its antibodies are highly potent in neutralizing currently circulating COVID-19 Omicron variants.
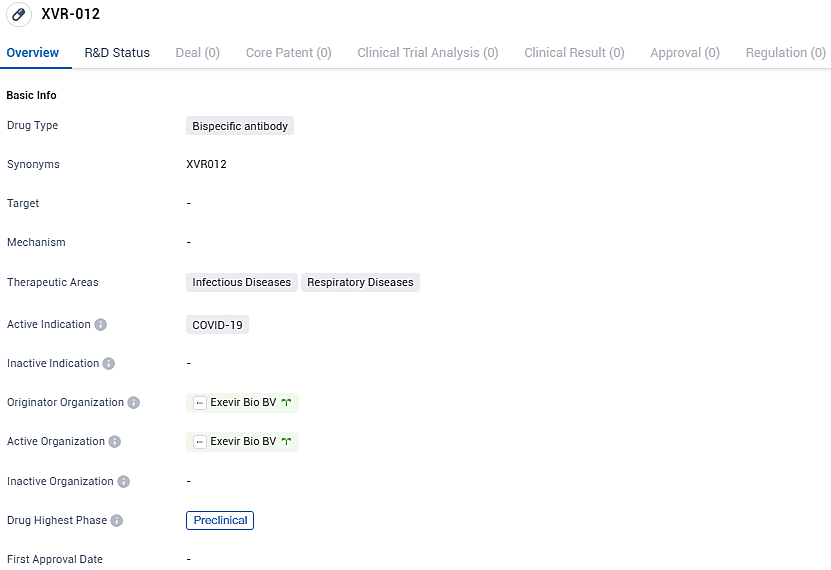
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
All authorized SARS-CoV-2 therapeutic antibodies that have been used in the clinic show severe to complete loss of virus neutralization potency against the currently circulating variants. ExeVir’s XVR012, a cocktail of XVR013m and XVR014, targets three distinct epitopes highly conserved across the broad sarbecovirus subgenus of Coronaviridae to minimize the risk for viral escape.
XVR013m is a heavy chain-only antibody targeting a unique membrane-proximal epitope in the S2 subunit of the spike protein. XVR014 is a bispecific heavy chain-only antibody that, complementary to XVR013m, targets two epitopes in the receptor-binding domain of the S1 subunit of the spike protein. XVR013m and XVR014 neutralize both SARS-CoV-1 and SARS-CoV-2 viruses.
The cocktail of XVR012 demonstrates exceptional neutralization potency, with an IC50 range of 0.8 to 1.8 ng/mL (pseudovirus neutralization assay) against all SARS-CoV-2 variants evaluated so far, including the currently highly prevalent E.G.5.1, HV.1 and BA.2.86.1. The activity against new circulating variants under monitoring is continually being tested.
Dr. Torsten Mummenbrauer, CEO of ExeVir, said: “Our unique and powerful approach of targeting three highly conserved epitopes in the SARS-CoV-2 spike protein with our cocktail XVR012 is anticipated to be highly potent against virtually all currently circulating variants under monitoring, including EG.5.1, HV.1, and BA.2.86.1. There is still a strong need for novel antibodies that work against the newest Omicron variants, and these new data demonstrate that ours can be part of the solution.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
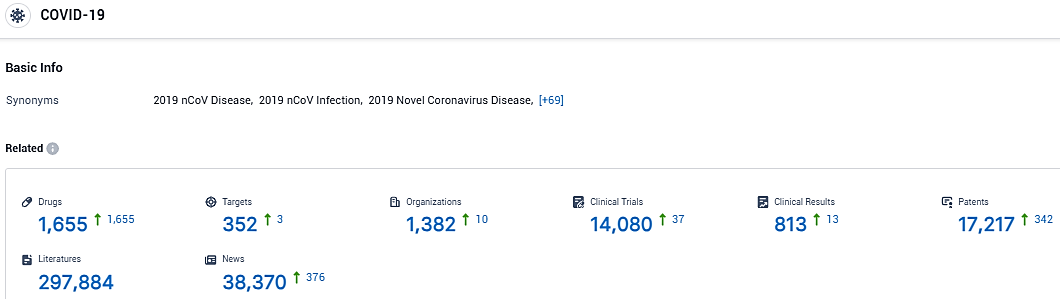
According to the data provided by the Synapse Database, As of January 16, 2024, there are 1655 investigational drugs for COVID-19, including 352 targets, 1382 R&D institutions involved, with related clinical trials reaching 14080, and as many as 17217 patents.
XVR-012 is a bispecific antibody being developed by for the treatment of COVID-19. It is currently in the preclinical phase, and its therapeutic areas include infectious diseases and respiratory diseases. Further research and development are required to determine the drug's potential effectiveness and safety in human clinical trials.