Amgen's Maritide Shows Significant Weight Loss in Phase 2 Trial
Amgen (NASDAQ:AMGN) has reported favorable findings at 52 weeks from a double-blind, dose-ranging Phase 2 trial involving MariTide (maridebart cafraglutide, previously known as AMG 133), which is a novel antibody-peptide conjugate given subcutaneously on a monthly basis or less. In individuals struggling with obesity or who are overweight but do not have Type 2 diabetes, MariTide showed an average weight loss of approximately 20% at the 52-week mark, without reaching a plateau, suggesting the possibility of continued weight reduction beyond this period.
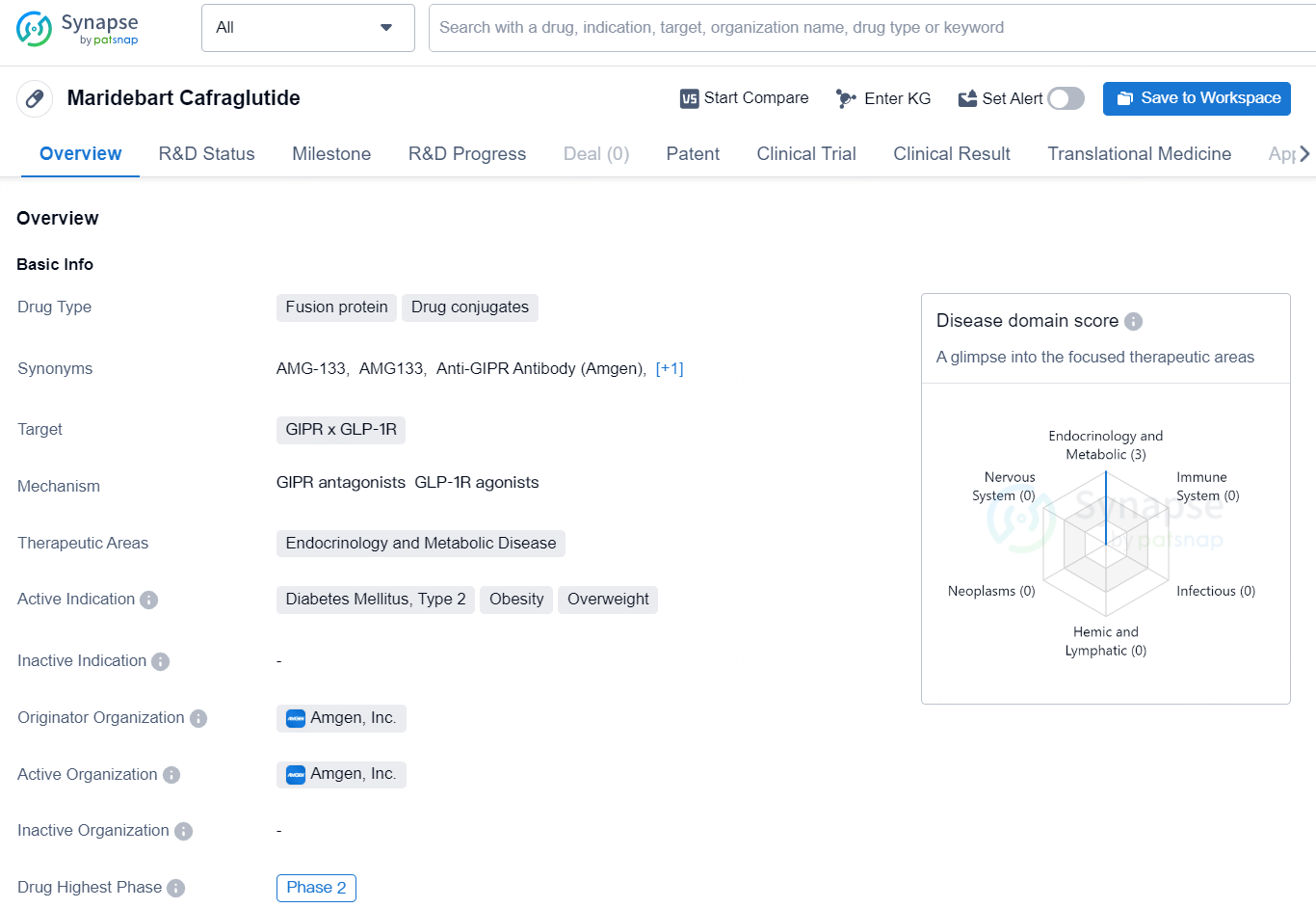
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The research indicated that individuals with obesity or overweight along with Type 2 diabetes generally experience smaller weight reductions when treated with GLP-1 therapies. However, they managed to achieve an average weight loss of approximately 17%, without encountering a plateau, and reduced their average hemoglobin A1C (HbA1c) levels by as much as 2.2 percentage points by the 52nd week. In conclusion, a weight loss plateau was absent in both study cohorts, suggesting that further weight reduction is possible beyond the 52-week mark.
MariTide also revealed significant and clinically relevant enhancements in cardiometabolic measures, such as blood pressure, triglycerides, and high-sensitivity C-reactive protein (hs-CRP), across various dosing levels. No notable increases in free fatty acids were recorded.
Moreover, no correlation was found between MariTide administration and changes in bone mineral density.
The Phase 2 trial primarily reported gastrointestinal (GI) adverse events (AEs), which included symptoms like nausea, vomiting, and constipation. Most nausea and vomiting cases were mild, temporary, and mainly linked to the initial dose. The frequency of these symptoms significantly decreased with dose escalation. In the dose escalation groups, those who experienced these symptoms found them to be episodic, typically resolving within a median of six days for nausea and one to two days for vomiting. The rate of discontinuation due to any adverse event in the dose escalation groups was approximately 11%, with GI-related events accounting for less than 8%. No additional safety concerns were noted. A separate ongoing Phase 1 pharmacokinetic study is evaluating various dosing regimens as part of a preliminary analysis.
“We are enthusiastic about the unique profile of MariTide, which showcases clinically significant features including substantial and continuous weight reduction, dosing every month or less often, notable enhancements in cardiometabolic metrics, and a strong decrease in HbA1C,” stated Jay Bradner, M.D., executive vice president of Research and Development and chief scientific officer at Amgen. “These findings bolster our confidence in launching MARITIME, a Phase 3 initiative addressing obesity and several related conditions, offering a distinct potential treatment avenue for patients.”
Data from this Phase 2 study will be shared at an upcoming medical conference and submitted for publication.
The ongoing second part of the Phase 2 trial is examining MariTide's effects beyond the 52-week mark to assess continued weight loss with ongoing treatment, weight stabilization through less frequent or lower doses, and the sustainability of weight loss after stopping MariTide. Over 90% of eligible participants opted to continue in the second phase of the study.
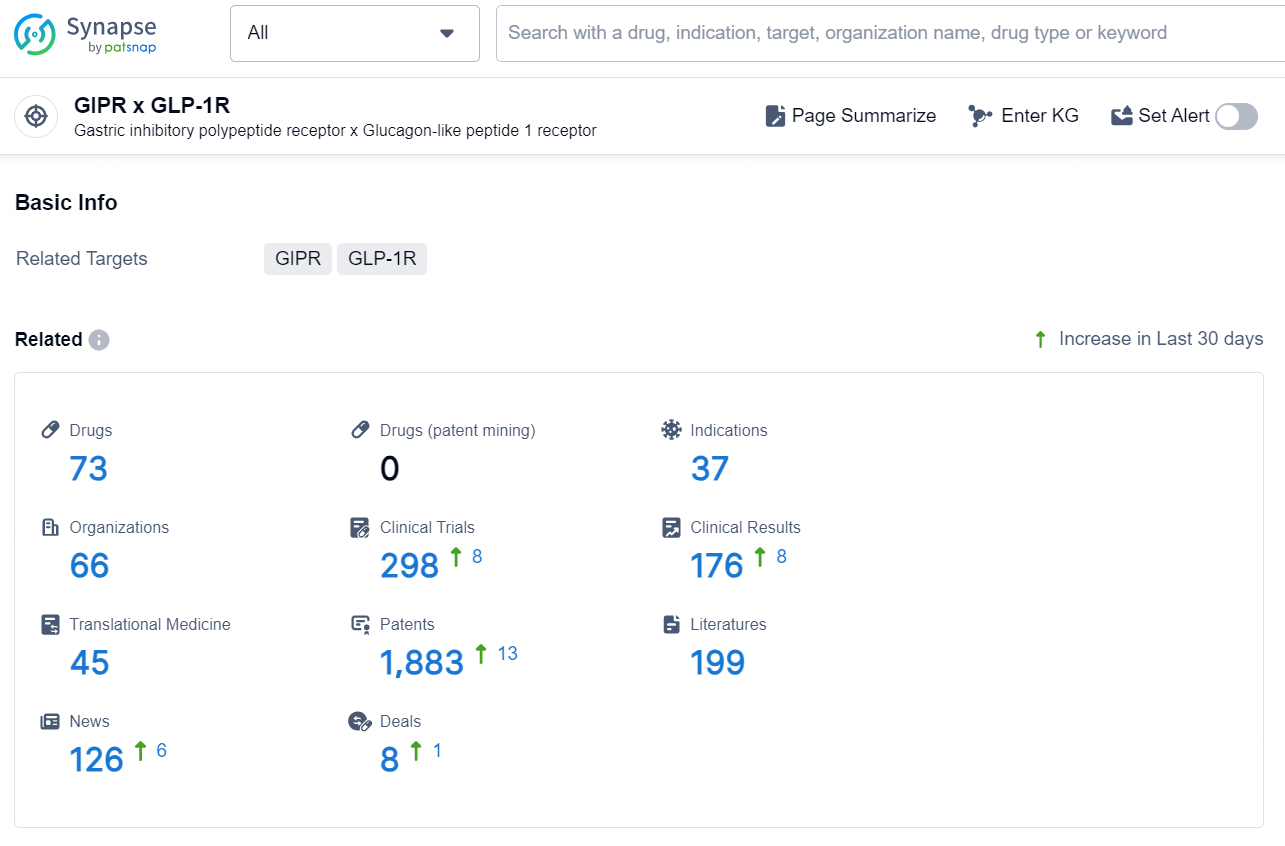
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of December 4, 2024, there are 73 investigational drugs for the GIPR x GLP-1R target, including 37 indications, 66 R&D institutions involved, with related clinical trials reaching 298, and as many as 1883 patents.
Maridebart Cafraglutide is a fusion protein drug conjugate targeting GIPR x GLP-1R. It falls within the therapeutic areas of Endocrinology and Metabolic Disease, with its active indications being Diabetes Mellitus, Type 2, Obesity, and Overweight. The drug is developed by the pharmaceutical company, Amgen, Inc.