SCG Cell Therapy Presents Promising Phase 1 Results for HBV TCR-T Treatment at AASLD 2024
SCG Cell Therapy Pte Ltd (SCG), a biotechnology firm at the clinical trial stage specializing in pioneering immunotherapies for infectious diseases and related cancers, has released significant updates from its Phase 1 study of SCG101. This first-in-class autologous T-cell receptor-engineered T cell (TCR-T) therapy is specifically designed to target hepatitis B surface antigen (HBsAg) in patients with hepatitis B virus (HBV). These findings were showcased at the 2024 AASLD Liver Meeting held in San Diego, USA.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
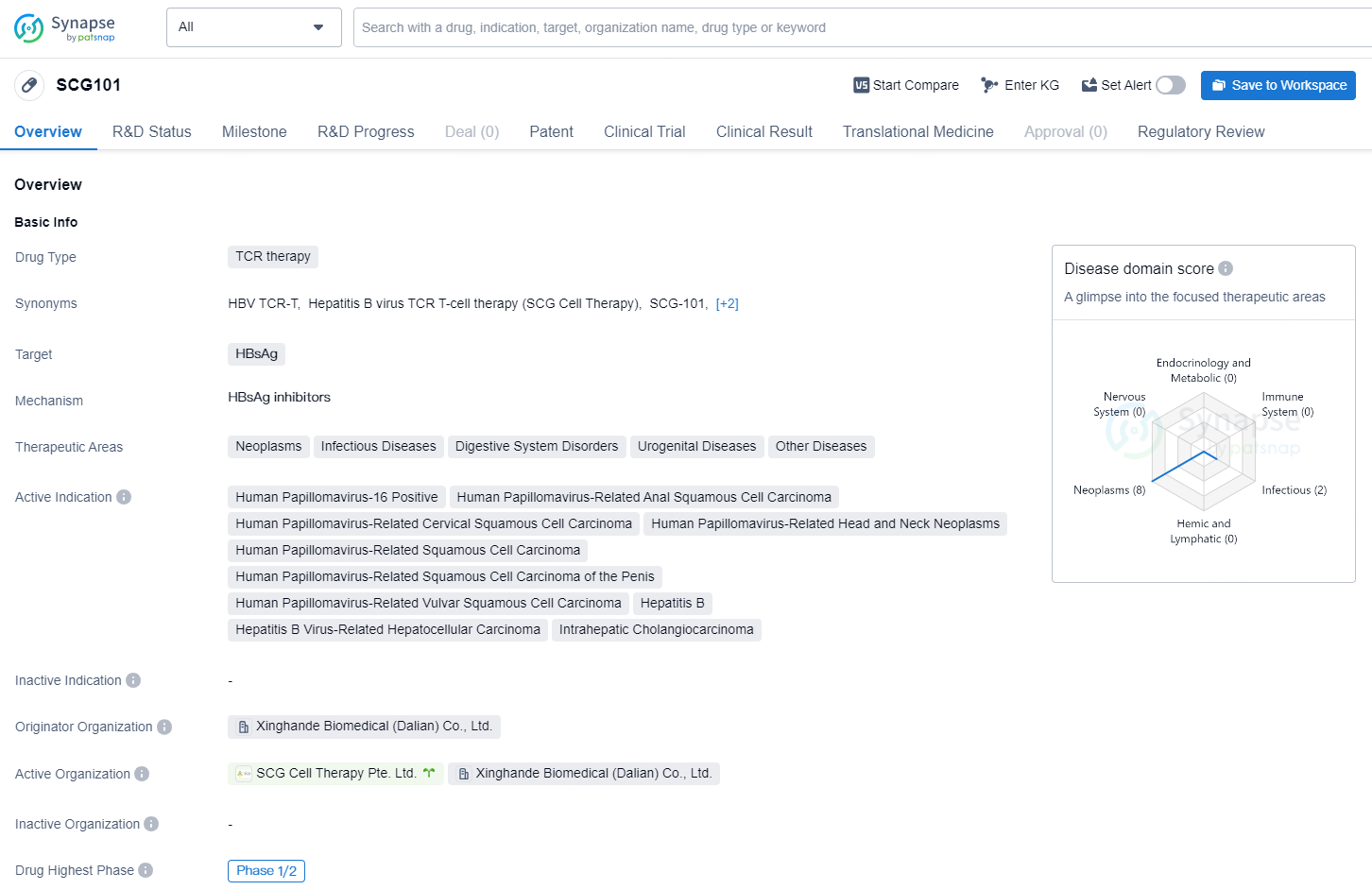
In a Phase 1 clinical trial, SCG101 showed encouraging antiviral effects in individuals with advanced HBV-related hepatocellular carcinoma (HBV-HCC). In a group of 12 patients receiving a single intravenous dose of SCG101 at a concentration of between 5.0×10^7 and 1.0×10^8 TCR+ T cells/kg, all participants presented a notable decrease in serum HBsAg levels. Specifically, 11 out of the 12 patients (92%) experienced a drop of 1.0 to 4.6 log10, and these levels remained below 100 IU/mL for the duration of the follow-up, which extended to one year. Remarkably, four patients (33%) achieved complete HBsAg clearance within 21 days post-infusion, with this loss remaining stable during follow-up.
The safety profile of SCG101 was found to be generally positive, with good tolerability noted among the subjects. The most frequently reported treatment-related adverse effects were temporary increases in liver enzymes, fever, cytopenia, cytokine release syndrome (CRS), low albumin levels, and low sodium levels. These side effects align with the mechanism of action of SCG101, which focuses on HBsAg-targeted immune stimulation leading to the removal of diseased hepatocytes and HCC cells.
Hepatitis B virus (HBV) continues to pose a significant global health challenge, impacting over 250 million individuals around the world. It is a primary contributor to liver cancer, being responsible for 50% to 80% of hepatocellular carcinoma cases globally. Chronic HBV infection results in the incorporation of HBV DNA into the host’s genome, leading to ongoing HBsAg production, chromosomal instability, and oncogene activation, all of which facilitate the onset of hepatocellular carcinoma. SCG101 aims to target a particular HBV peptide found on infected cells and on hepatocytes with integrated HBV DNA. It employs both cytolytic and non-cytolytic pathways to effectively eradicate HBV-infected hepatocytes as well as premalignant and HBV-HCC cells that possess HBV-DNA integration.
"The favorable results from our Phase 1 trial represent a significant advancement in the development of SCG101, our pioneering HBV-specific TCR-T therapy. These findings not only highlight SCG101's potential to generate substantial and lasting antiviral effects in patients but also emphasize our distinctive strategy in HBV-specific TCR T cell therapy. We are optimistic about these initial outcomes and are dedicated to progressing SCG101 through further clinical trials as we aim to offer new therapeutic alternatives for patients grappling with this difficult condition,” stated Christy Ma, CEO of SCG Cell Therapy. "We are confident that our GianTTM TCR discovery platform can tackle a wide range of infection-related cancers, including those associated with human papillomavirus (HPV) leading to cervical cancer and head and neck malignancies, as well as Epstein-Barr virus (EBV) linked nasopharyngeal carcinoma and gastric cancer, thereby establishing a new framework for immune-based cancer treatments.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
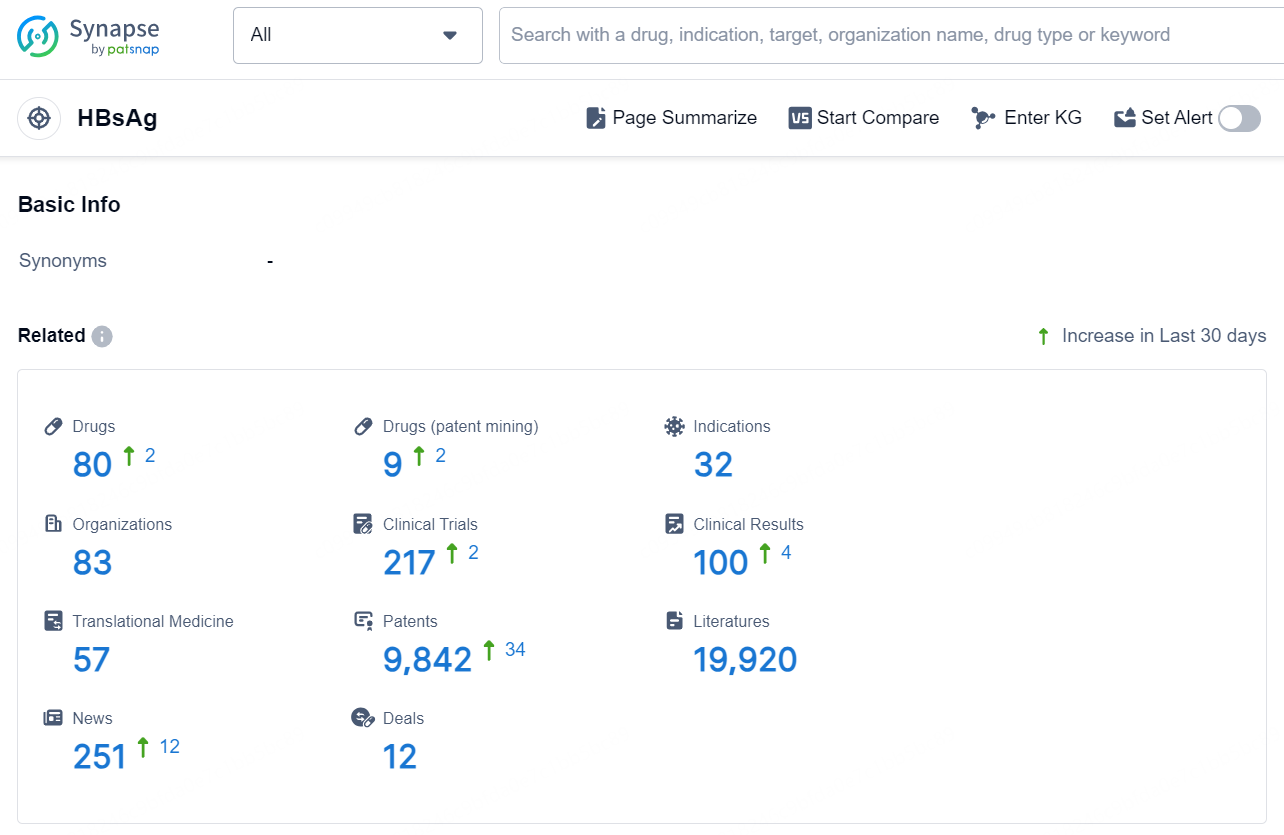
According to the data provided by the Synapse Chemical, As of December 2, 2024, there are 80 investigational drugs for the HBsAg target, including 32 indications, 83 R&D institutions involved, with related clinical trials reaching 217, and as many as 9842 patents.
SCG101 is a TCR therapy drug developed by Xinghande Biomedical (Dalian) Co., Ltd. It is designed to target HBsAg and is being developed for the treatment of various medical conditions in the therapeutic areas of neoplasms, infectious diseases, digestive system disorders, urogenital diseases, and other diseases.