PTC Therapeutics Reports Key Findings from CardinALS Study on Utreloxastat for ALS Patients
PTC Therapeutics, Inc. (NASDAQ: PTCT) has reported that its global Phase 2 placebo-controlled CardinALS trial failed to achieve its main objective of demonstrating a reduction in disease progression based on the combined ALSFRS-R and mortality assessment. Although there was a slight numerical advantage noted regarding the primary endpoint, as well as a relationship between the positive clinical effects and decreased levels of plasma neurofilament light chain (NfL), a marker for neuronal injury, the results did not reach statistical significance (p= 0.52). Furthermore, the trial also did not attain significance for the secondary efficacy measures.
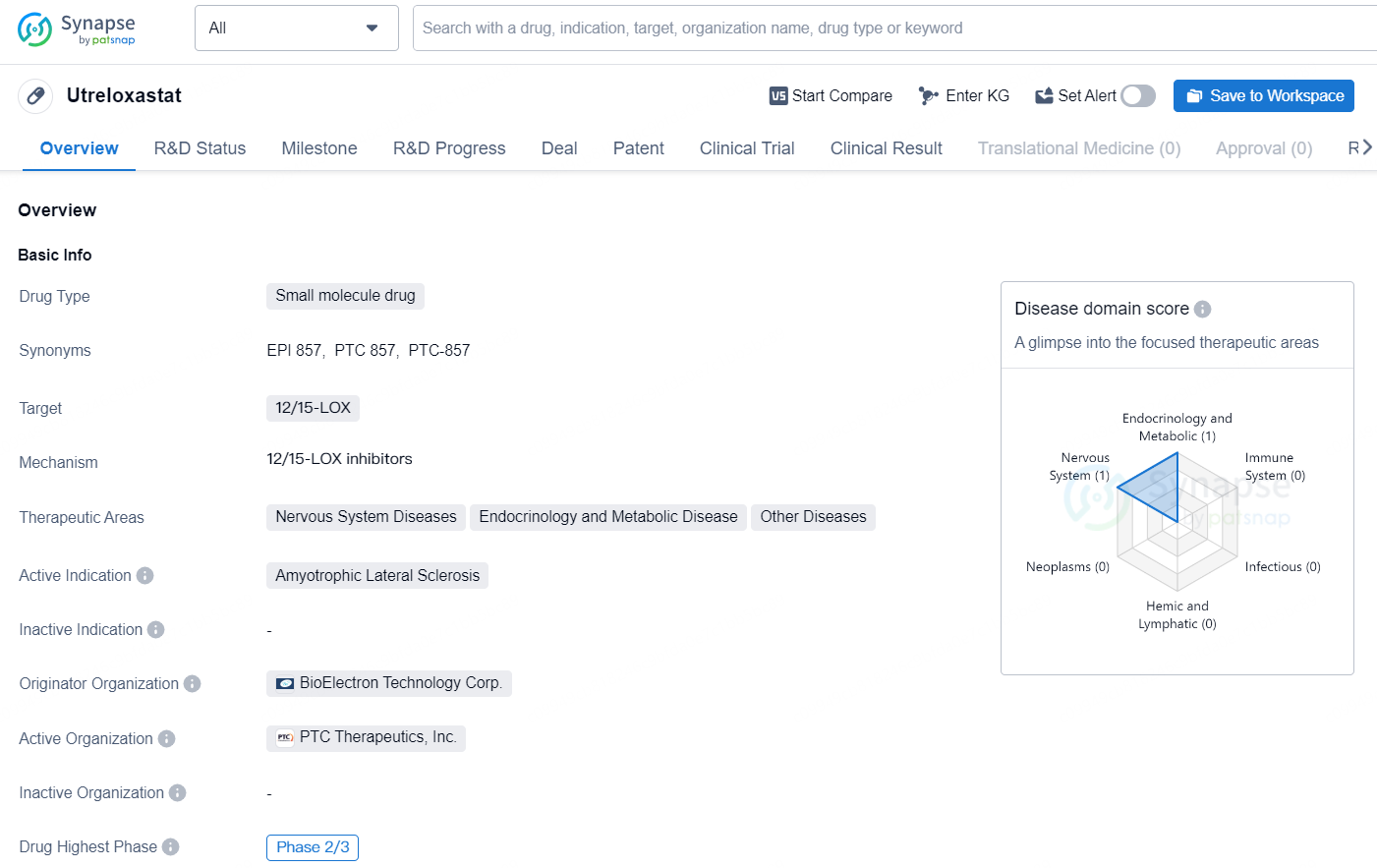
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"We express our gratitude to all patients, their families, and the healthcare providers who engaged in the CardinALS trial," stated Matthew B. Klein, M.D., CEO of PTC Therapeutics. "Naturally, we are disappointed that we could not demonstrate the effectiveness of the treatment or offer a viable option to meet the substantial unmet medical needs of ALS patients."
In the CardinALS trial, Utreloxastat was shown to be safe and generally well tolerated. Nevertheless, due to the absence of efficacy data and biomarker indicators, there are currently no plans for further development.
Amyotrophic lateral sclerosis, commonly known as ALS, motor neuron disease, or Lou Gehrig’s disease, is a rare and progressive neurodegenerative condition that is ultimately fatal. It impacts motor neurons in both the brain and spinal cord. Individuals with ALS face life-threatening challenges as they gradually lose their ability to move, speak, eat, and breathe.
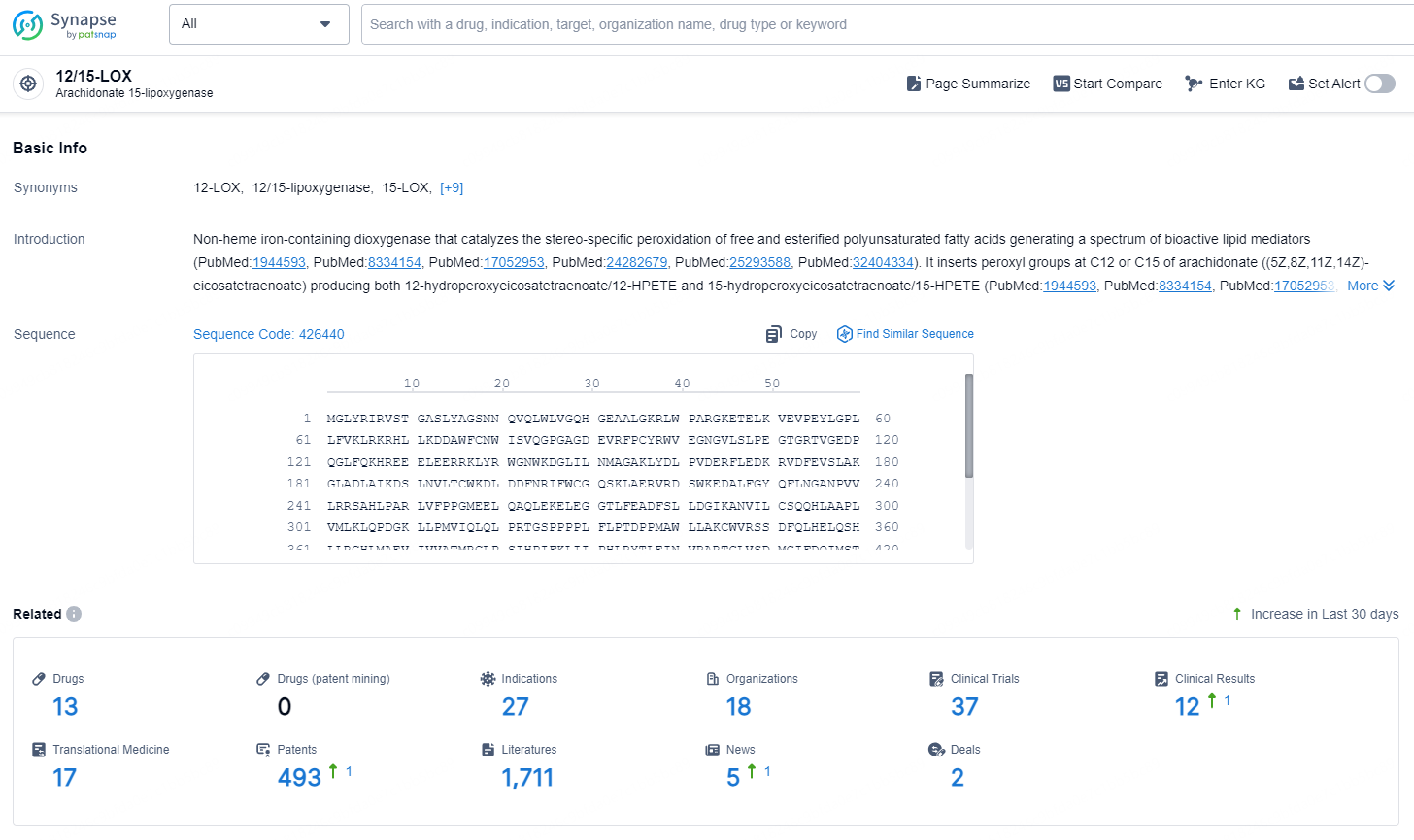
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 4, 2024, there are 13 investigational drugs for the 12/15-LOX target, including 27 indications, 18 R&D institutions involved, with related clinical trials reaching 37, and as many as 493 patents.
Utreloxastat is a small molecule drug that targets 12/15-LOX and is being developed by BioElectron Technology Corp. The drug is currently in the Phase 2/3 stage of development, with the highest global phase being Phase 2/3. Utreloxastat is intended for the treatment of Amyotrophic Lateral Sclerosis, a nervous system disease.