Amicus Therapeutics Declares FDA Sanction and Introduction of Novel Therapy for Pompe Disease
Amicus Therapeutics has confirmed that the U.S. FDA has given the green light to Pombiliti™ (cipaglucosidase alfa-atga) + Opfolda™ (miglustat) 65mg capsules. This dual-component treatment is recommended for mature individuals who suffer from late-onset Pompe disease, weigh equal to or more than 40 kg, and are not showing progress with their ongoing enzyme replacement therapy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Pompe disease is a scanty, incapacitating, and fatal lysosomal disorder triggered by the insufficiency of the acid alpha-glucosidase enzyme. Lowered GAA levels result in excessive glycogen storage within lysosomes of muscle cells, leading to muscle damage. The disorder's intensity varies, with major manifestations like loss of muscle strength and gradual respiratory issues.
Pombiliti is a natural human GAA enzyme, expressed with elevated bis-M6P amounts, created to enhance intake into muscle cells. After its entry into the cell, Pombiliti can properly convert to its most active and fully formed structure to reduce glycogen. Opfolda, on the other hand, is designed to stabilize the enzyme in the blood.
The FDA's approval for Pombiliti and Opfolda underscores the influence of scientific research and medicine, as well as our powerful persistence to enhance the quality of life for individuals grappling with Pompe disease, expressed John F. Crowley, Executive Chairman of Amicus Therapeutics, Inc. He added, "I am extraordinarily proud of our team and profoundly thankful to those involved in achieving this drug approval."
Bradley Campbell, the President and CEO of Amicus Therapeutics, Inc., declared the FDA's endorsement of Pombiliti and Opfolda as a significant landmark. He said, "Our highly competent crew is set to introduce this medication in the U.S., and we aspire to quickly provide this novel treatment approach to all eligible adults affected by late-onset Pompe disease and are not showing improvement with their ongoing ERT."
Amicus Therapeutics will launch Pombiliti + Opfolda in the U.S. The FDA had earlier accorded a Breakthrough Therapy designation for this combo. It has also gained approval for treating adults with LOPD in both the European Union and the United Kingdom.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
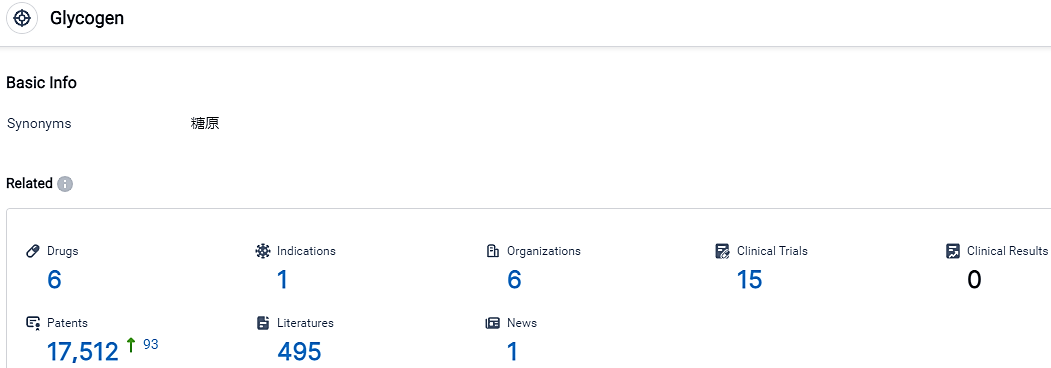
According to the data provided by the Synapse Database, As of October 8, 2023, there are 6 investigational drugs for the Glycogen target, including 1 indications, 6 R&D institutions involved, with related clinical trials reaching 15,and as many as 17512 patents.
The approval of Cipaglucosidase alfa represents a significant advancement in the field of biomedicine and offers new treatment possibilities for patients with Glycogen Storage Disease Type II. The drug's originator organization, Amicus Therapeutics, Inc., has demonstrated their dedication to developing innovative therapies for rare genetic disorders, further contributing to the progress of the pharmaceutical industry.