Amylyx Reveals Key Findings from Worldwide Stage 3 PHOENIX Study on AMX0035 for ALS Treatment
Amylyx Pharmaceuticals, Inc. has disclosed preliminary findings from the PHOENIX study, which pertains to a 48-week international, randomized, double-blind, placebo-controlled Phase 3 trial, centering on the evaluation of AMX0035 for patients diagnosed with amyotrophic lateral sclerosis.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The PHOENIX trial did not achieve its primary goal, as there was no significant statistical improvement noted in the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale total score after 48 weeks from the initial benchmark. Similarly, no significant findings were observed for the secondary outcomes being measured. Amylyx is preparing to detail the findings from the PHOENIX study at a forthcoming healthcare conference and intends to document the study's findings in a scientific periodical before the year's end.
Amylyx is committed to ongoing discussions with healthcare regulatory bodies and the wider ALS network, encompassing medical specialists in ALS care, interdisciplinary healthcare providers, individuals with ALS, and their supporters. The aim is to thoroughly review the PHOENIX findings within the upcoming two months to guide future actions.
The company is currently evaluating the future path for RELYVRIO/ALBRIOZA in managing ALS, which might involve considering the option of a voluntary recall from the market. However, for now, RELYVRIO/ALBRIOZA and its associated patient assistance initiatives remain accessible for those affected by ALS. Amylyx has chosen to halt the marketing efforts for this medication during this period.
Justin Klee and Joshua Cohen, the Co-CEOs of Amylyx, expressed their unforeseen disappointment with the outcomes of PHOENIX, especially after the promising results from the CENTAUR study. They reaffirmed their dedication to serving the ALS community and pursuing their mission. This includes the continued development of AMX0035, which has shown promising signs in treating neurodegenerative diseases like Wolfram syndrome and progressive supranuclear palsy, as well as advancing AMX0114, a drug in development that uses antisense oligonucleotide technology to inhibit calpain-2, for potential use in ALS treatment.
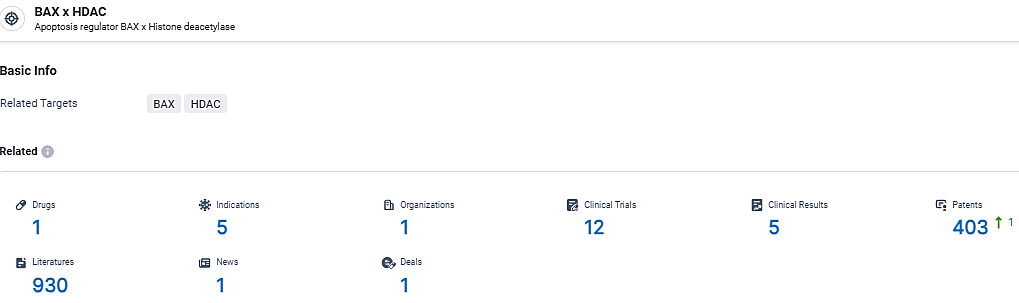
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 11, 2024, there are 1 investigational drugs for the BAX and HDAC target, including 5 indications,1 R&D institutions involved, with related clinical trials reaching 12, and as many as 403 patents.
AMX0035 has been approved for use in Canada and has shown potential in treating various diseases and conditions within the nervous system, endocrinology and metabolic disease, eye diseases, immune system diseases, congenital disorders, otorhinolaryngologic diseases, and urogenital diseases. The drug targets BAX and HDAC and has active indications for Amyotrophic Lateral Sclerosis, Supranuclear Palsy, Progressive, and Wolfram Syndrome. Its regulatory support includes priority review, conditional marketing approval, and orphan drug designation.