An analysis of Anlotinib Dihydrochloride's R&D progress and its clinical results presented at the 2023 ESMO_ASIA Annual Meeting
Advanced gastrointestinal (GI) tumors, such as colorectal, gastric and pancreatic cancers (CRC, GC, and PC), and esophageal squamous cell carcinoma (ESCC), 20%-50% with liver metastases (LMs) have a poor prognosis. Previous trials showed that anlotinib plus chemotherapy has promising clinical activity and a tolerable safety profile for advanced CRC and ESCC, especially with LMs. And the latest clinical results of Anlotinib Dihydrochloride were presented in 2023 ESMO_ASIA.
Anlotinib Dihydrochloride's R&D Progress
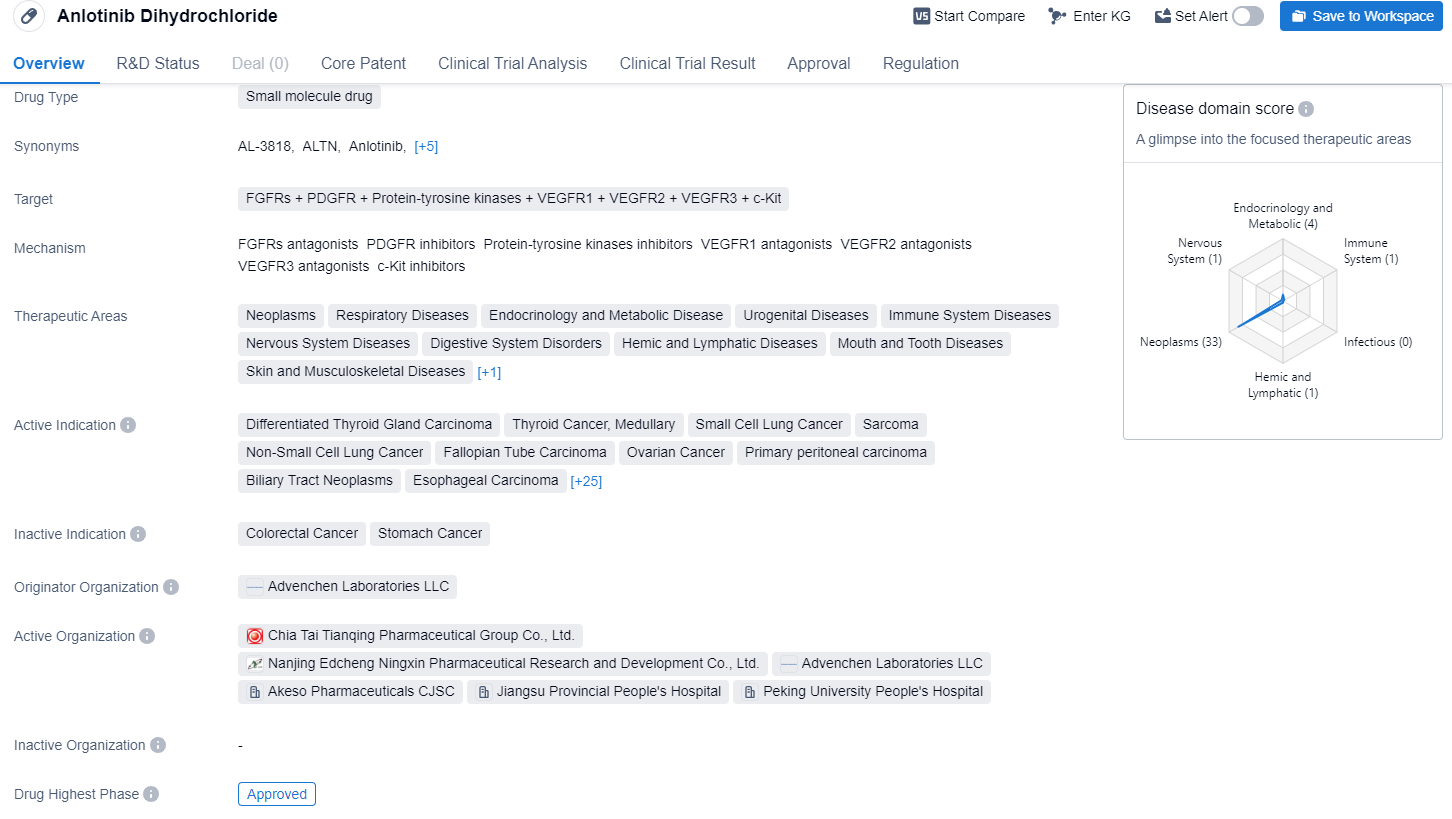
Anlotinib Dihydrochloride is a small molecule drug that targets various proteins including FGFRs, PDGFR, protein-tyrosine kinases, VEGFR1, VEGFR2, VEGFR3, and c-Kit. It has shown potential therapeutic benefits in a wide range of therapeutic areas including neoplasms, respiratory diseases, endocrinology and metabolic diseases, urogenital diseases, immune system diseases, nervous system diseases, digestive system disorders, hemic and lymphatic diseases, mouth and tooth diseases, skin and musculoskeletal diseases, and otorhinolaryngologic diseases.
According to the Patsnap Synapse, Anlotinib Dihydrochloride is developed by Advenchen Laboratories LLC. It has reached the highest phase of development, which is approved globally. And the clinical trial distributions for Anlotinib Dihydrochloride are primarily in the United States, China and United Kingdom. The key indication is Non-Small Cell Lung Cancer. 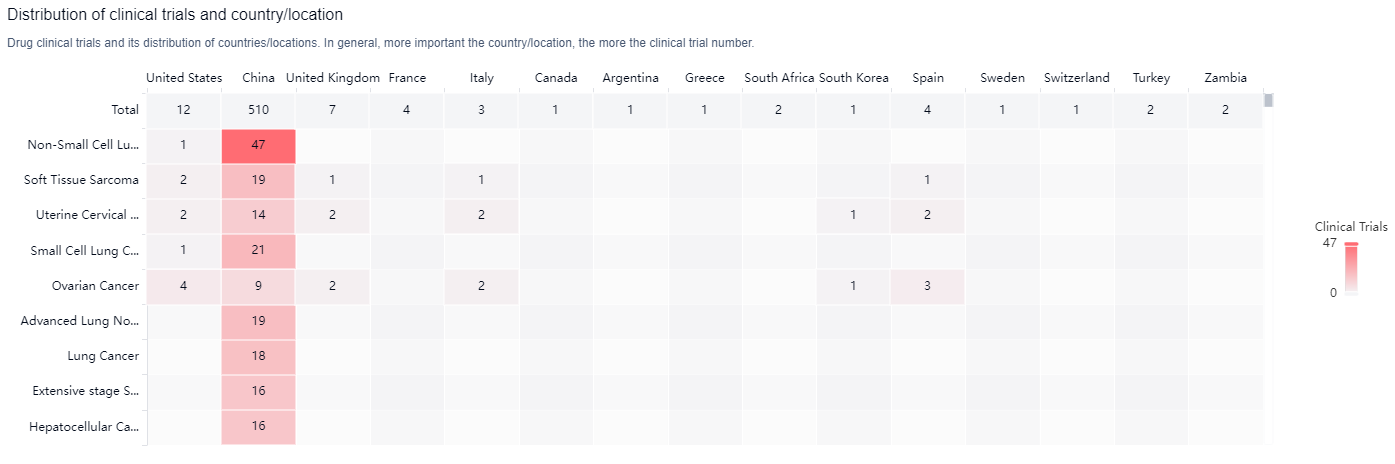
Detailed Clinical Result of Anlotinib Dihydrochloride
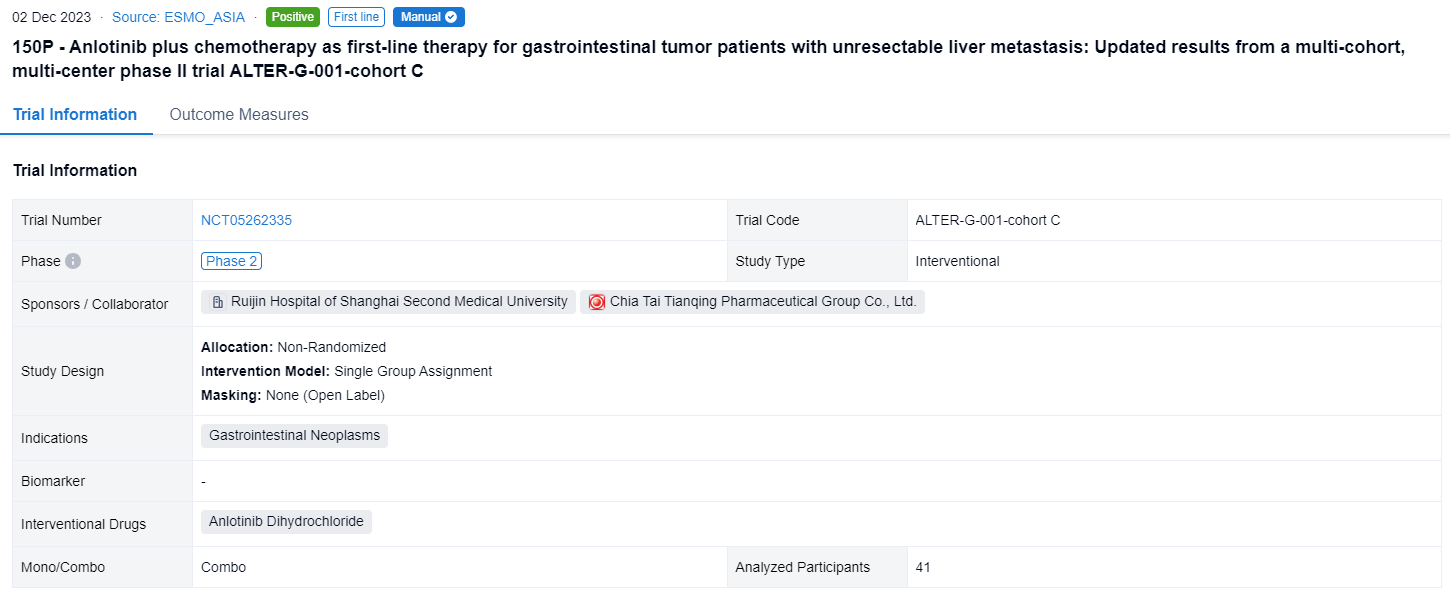
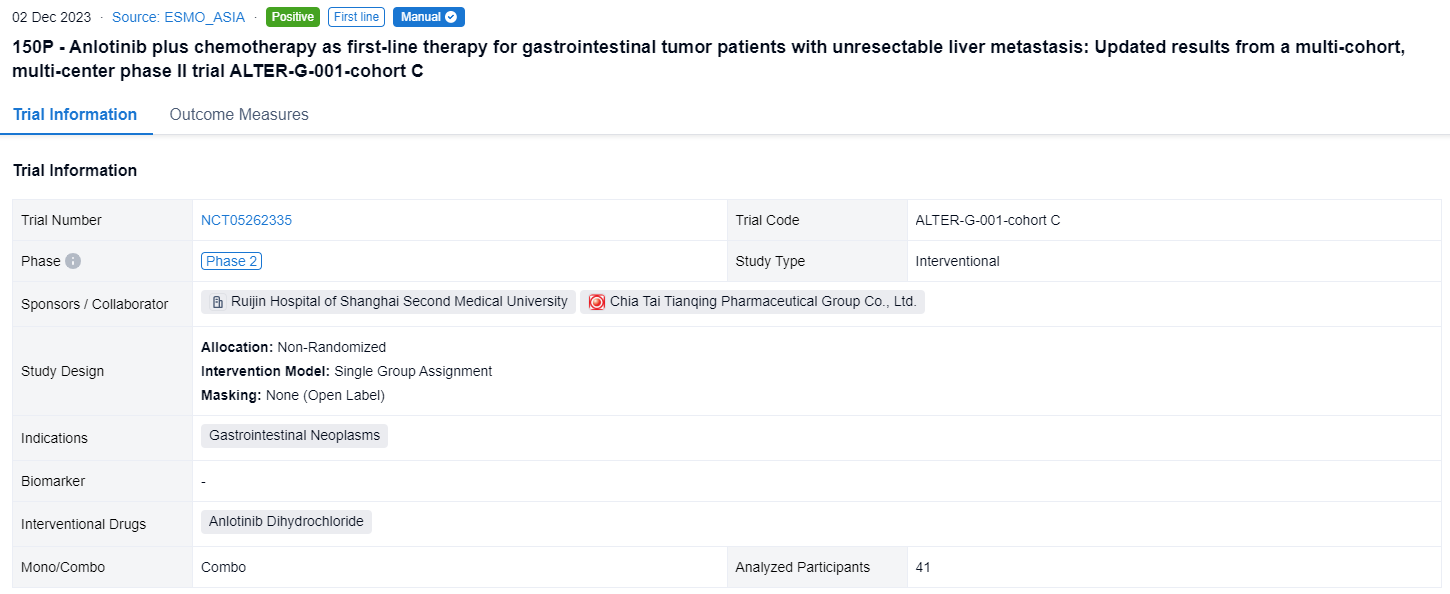
This non-randomized, single group assignment, open-labeled clinical trial (NCT05262335) was aimed to assess the efficacy and safety of anlotinib plus chemotherapy as first-line treatment for LMs GI tumors.
In this study, patients with unresectable LMs GI tumors and without previous systemic treatment would be divided into cohort A (CRC), cohort B (ESCC), and cohort C (other GI tumors, such as PC, GC, biliary tract cancer (BTC), etc.). In cohort C, patients received induction therapy: anlotinib plus standard chemotherapy. Patients without PD and radical resection received anlotinib and metronomic capecitabine (500 mg, PO, BID, days 1-21, q3w) maintenance until PD or unacceptable toxicity. The primary endpoint was ORR (RECIST 1.1). Secondary endpoints were DoR, PFS, OS, DCR, radical resection rate for LMs, and safety.
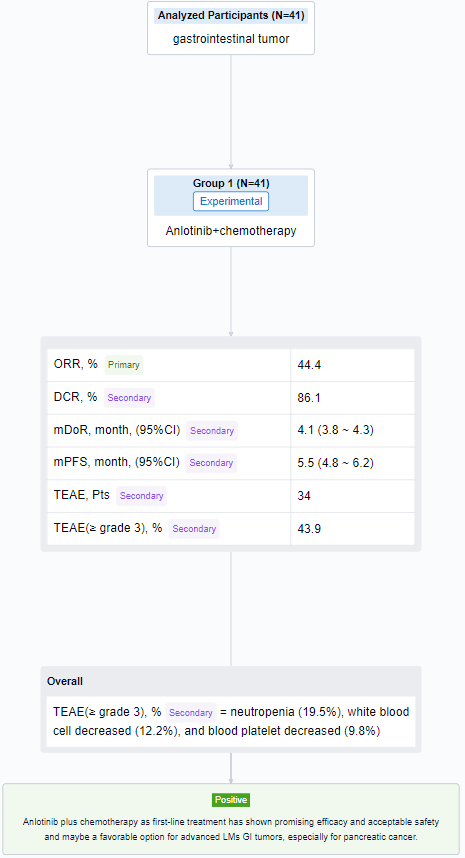
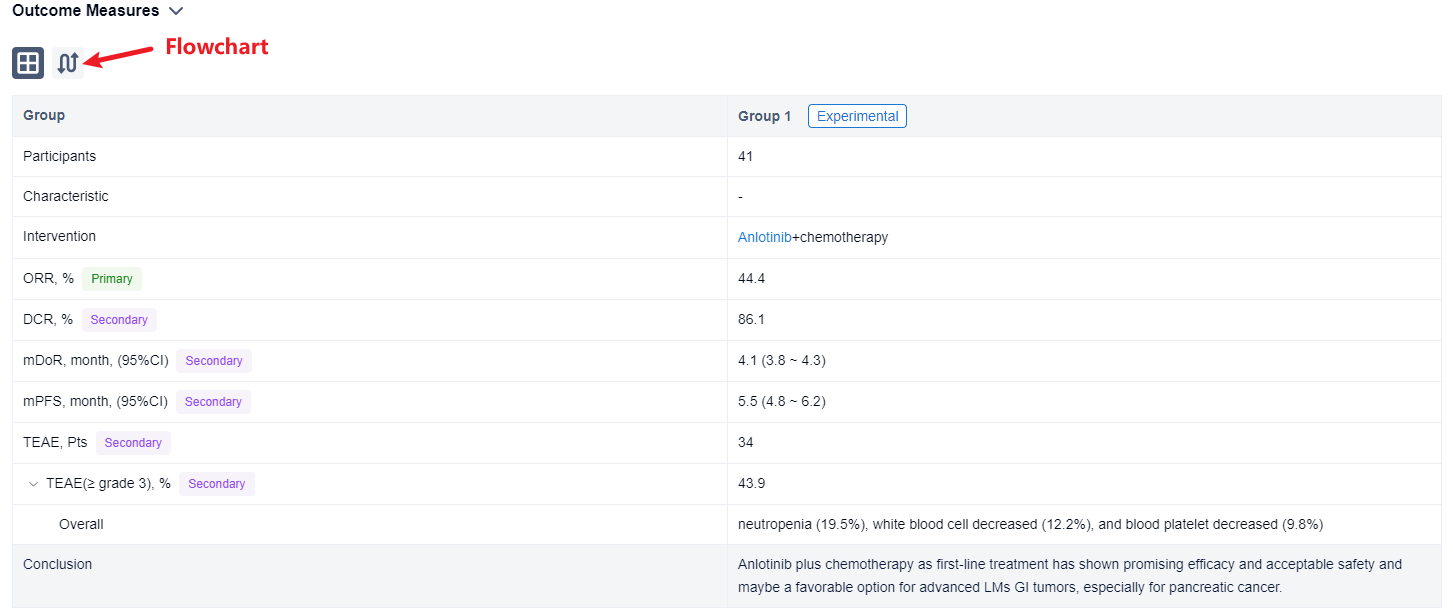
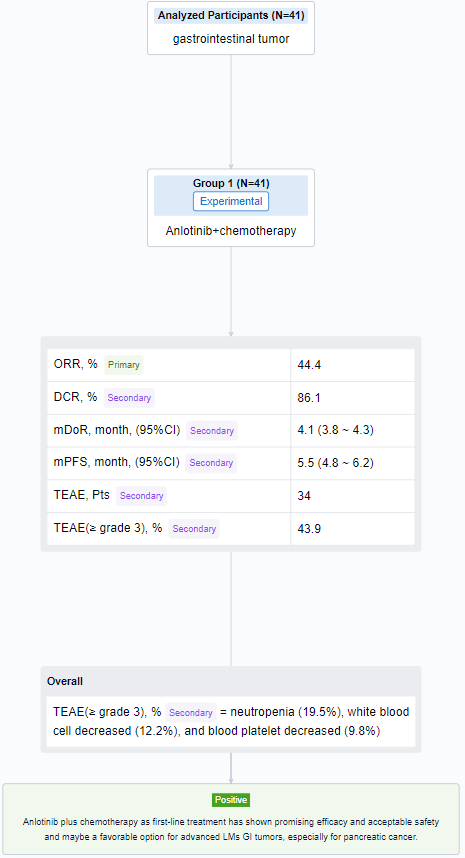
The result showed that as of August 14, 2023, 41 patients were enrolled in cohort C (29 PC, 6 GC, 5 BTC, and others), the median age was 64 years (34-74), 63.4% male, 92.7% ECOG-PS 1 and 56.1% had LMs only. After induction therapy, 4 patients (1 PC, 2 GC, 1 BTC) received surgical resection. Of 36 evaluable patients in cohort C, ORR and DCR were 44.4% and 86.1% (PR, n=16; SD, n=15, 12 SD had reduced tumor size). Of 24 evaluable pancreatic cancer patients, 11 had PR, 10 had SD, ORR and DCR were 45.8% and 87.5%. According to the Kaplan-Meier method, the median DoR was 4.1 months (95%CI, 3.8-4.3) and the median PFS was 5.5 months (95%CI, 4.8-6.2). 34 patients in cohort C had TEAEs and ≥ grade 3 TEAEs (43.9%) mainly included neutropenia (19.5%), white blood cell decreased (12.2%), and blood platelet decreased (9.8%).
It can be concluded that Anlotinib plus chemotherapy as first-line treatment has shown promising efficacy and acceptable safety and maybe a favorable option for advanced LMs GI tumors, especially for pancreatic cancer.
How to Easily View the Clinical Results Using Synapse Database?
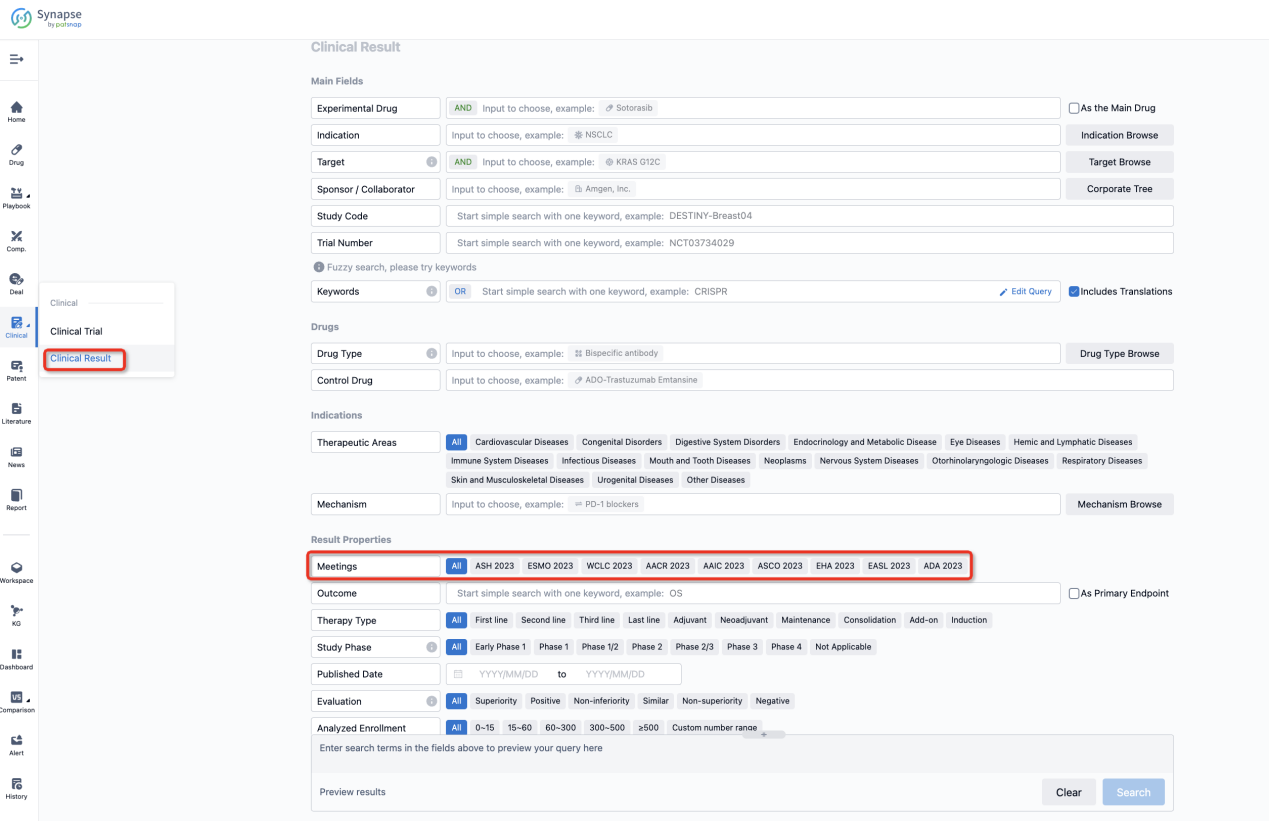
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
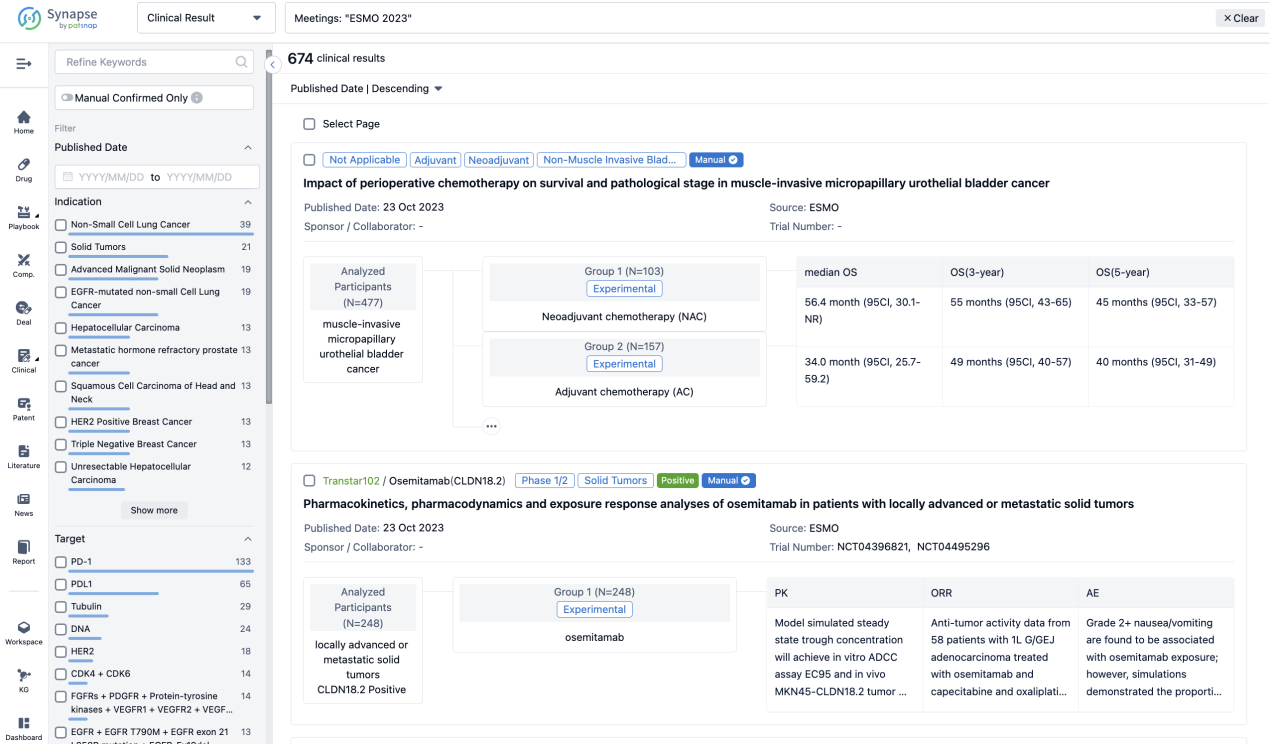
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
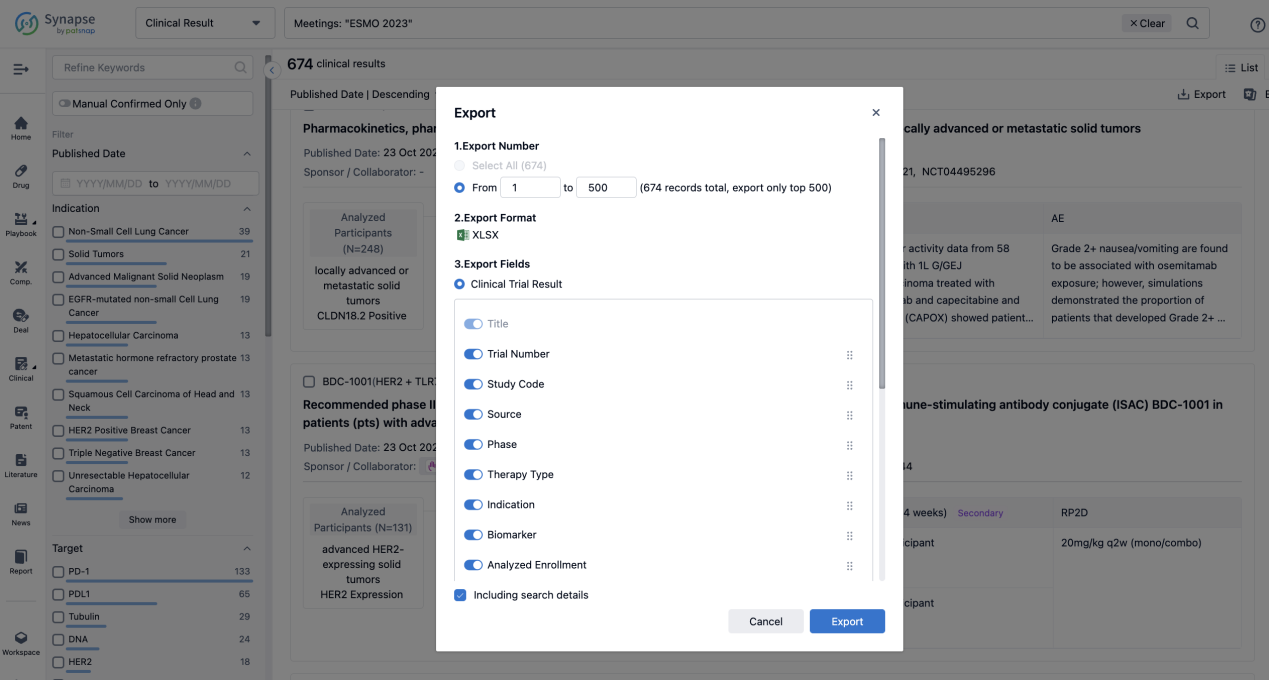
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!