What are PCSK9 inhibitors and how do you quickly get the latest development progress?
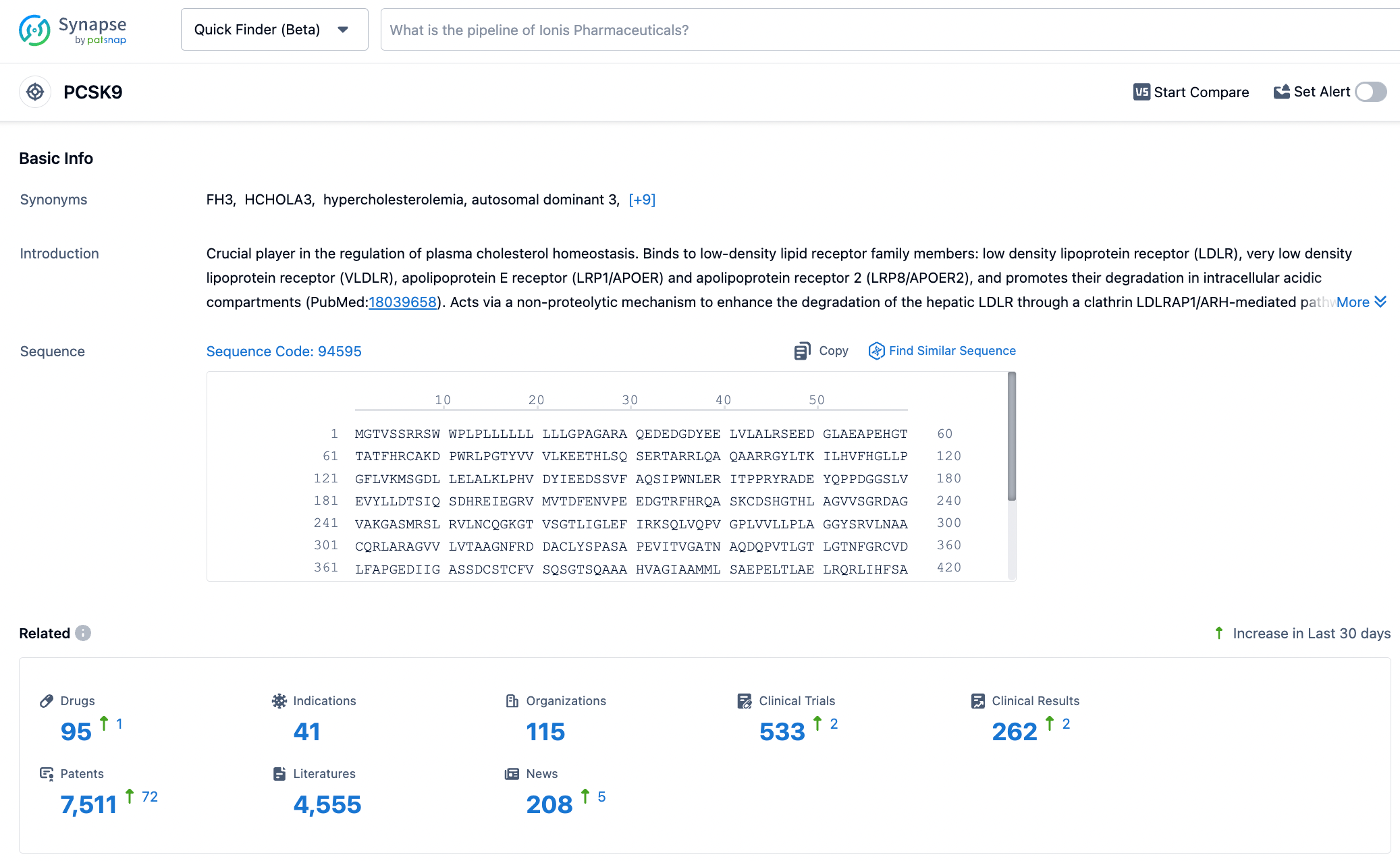
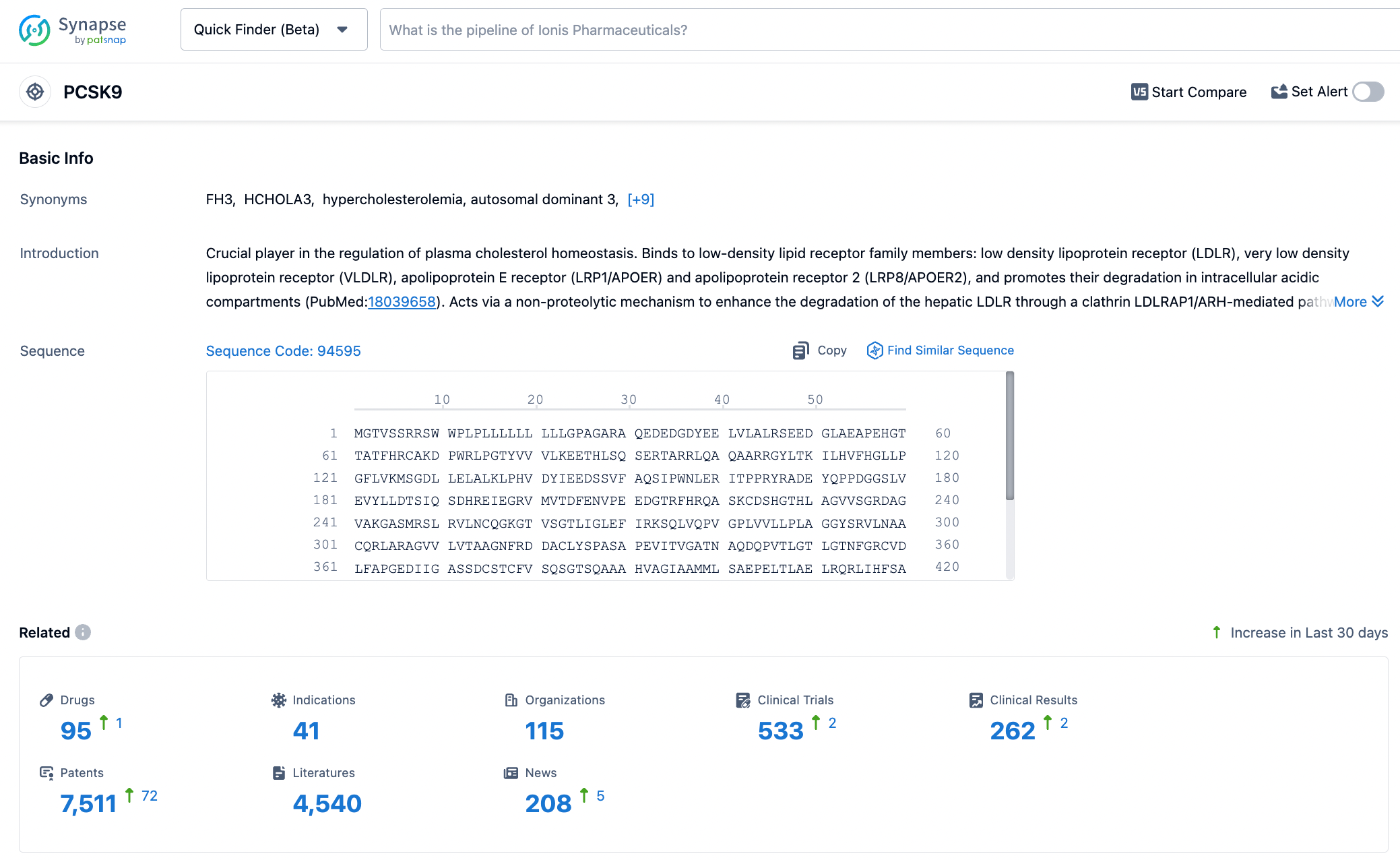
PCSK9, also known as Neural Apoptosis-Regulated Convertase 1 (NARC1), is the ninth major member of the proprotein convertase family, primarily derived from human liver cells, and is also expressed in the small intestine, renal interstitial cells, and neurons. The PCSK9 protein is composed of a signal peptide (containing 30 amino acids), a pro-domain (122 amino acids), a catalytic domain (299 amino acids), and a cysteine- and histidine-rich C-terminal domain (279 amino acids). To date, two genetic variants of PCSK9 have been identified: gain-of-function mutations and loss-of-function mutations. PCSK9 gain-of-function mutations are associated with familial hypercholesterolemia and cardiovascular diseases; conversely, individuals with loss-of-function mutations have lower levels of LDL-C and a reduced incidence of cardiovascular diseases. Click the image below to embark on a journey of PCSK9 discovery!
Most LDL particles are cleared through LDL receptors (LDL-R) on the surface of hepatocyte membranes. After LDL binds to LDL-R, the complex is internalized through endocytosis. Due to the decrease in pH, the LDL-R complex dissociates, releasing LDL-R back to the cell membrane surface to capture LDL particles again, further reducing plasma LDL levels. LDL particles are then degraded within lysosomes. If PCSK9 is present, an LDL and LDL-R-PCSK9 complex is formed, which is co-degraded within the lysosomes, including the LDL-R. This reduces the recirculation of LDL-R, decreases the clearance of LDL particles, and thereby increases plasma LDL levels.
PCSK9 inhibitors lower circulating LDL-C levels by blocking the degradation of LDL-R by PCSK9. According to their mechanism of action, they can be classified into three types: (1) Antisense oligonucleotides (ASO) or siRNA: These inhibit PCSK9 synthesis through gene silencing. (2) Monoclonal antibodies or mimetic antibody proteins: They directly inhibit the interaction between PCSK9 protein and LDL-R. (3) Peptides targeting the catalytic site of PCSK9: Such peptides can bind to and induce a conformational change in the catalytic site of PCSK9 protein, thereby affecting its interaction with LDL-R. (4) Small molecule inhibitors: Since the interaction interface between PCSK9 protein and LDL-R is relatively large and small molecules have limited contact area, the development of such drugs poses certain challenges.
PCSK9 inhibitors have displayed significant potential in the pharmaceutical market, primarily due to their use in treating hypercholesterolemia and cardiovascular diseases; as a breakthrough therapeutic approach, PCSK9 inhibitors are anticipated to continue their growth in the coming years, further enhancing the quality of life for patients.
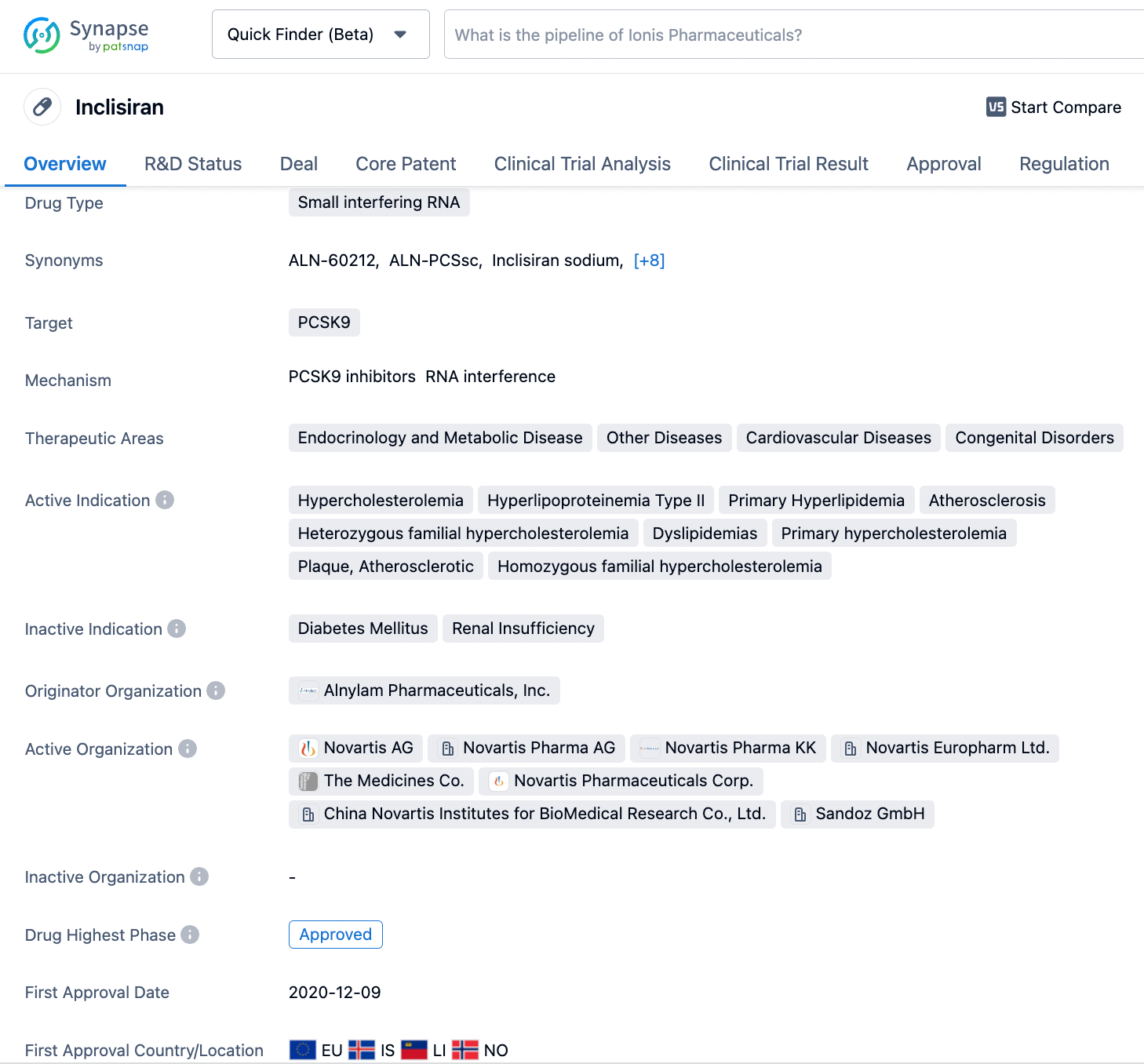
Key Drug: Inclisiran
Inclisiran (ALN-PCSsc), developed by Novartis, is a long-acting therapeutic agent that inhibits the synthesis of PCSK9 through RNA interference (RNAi) and requires only two subcutaneous injections per year. It was approved for marketing in December 2020. As the first siRNA drug targeting PCSK9, inclisiran features a specially designed GalNAc delivery system that first specifically binds to N-acetylgalactosamine (GalNAc) and the asialoglycoprotein receptor (ASGPR) on the surface of liver cell membranes, facilitating its entry into hepatocytes. Once inside the liver cells, inclisiran associates with the RNA-induced silencing complex (RISC) and, guided by the antisense strand, binds to the mRNA encoding PCSK9 protein, thereby inhibiting the production of PCSK9 protein.
On November 18, 2022, the Marketing Authorization Application (MAA) for Novartis's inclisiran injection was accepted by the National Medical Products Administration (NMPA) of China. In a prospective, non-interventional cohort study, the effectiveness of inclisiran in treating adults with primary hypercholesterolemia or mixed dyslipidemia was assessed. The results showed that out of 34 patients, 28 (82.4%) had their LDL-C levels measured on day 90±14 after the first injection of inclisiran, with an average reduction in LDL-C levels of 40.2% from baseline. Regarding safety, Chinese patients tolerated inclisiran well, with no adverse events leading to discontinuation of the drug or serious adverse events related to inclisiran reported.
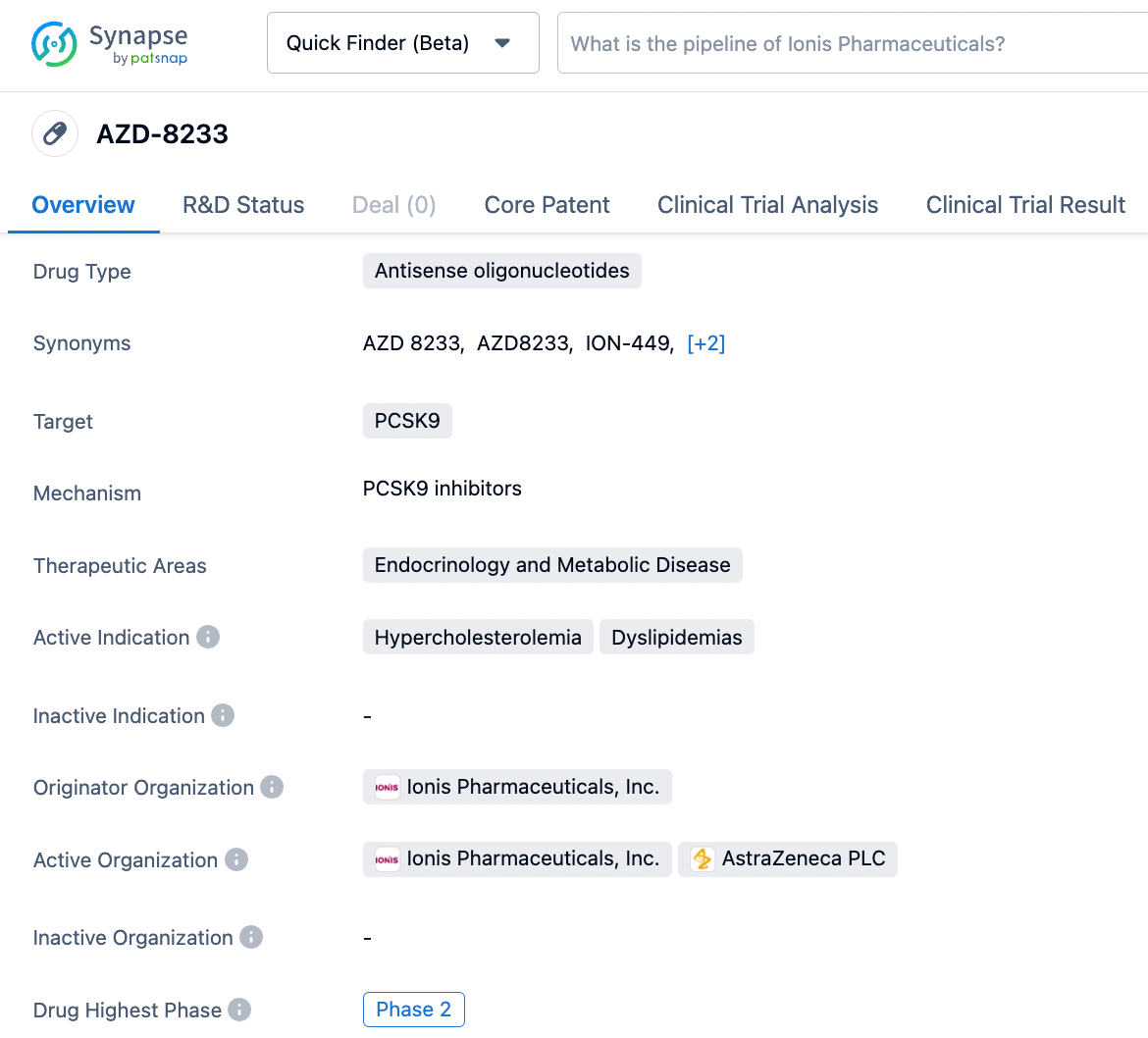
AstraZeneca: AZD8233
AZD8233 (ION449) is an antisense oligonucleotide (ASO) drug developed using the Ionis Ligand-Conjugated Antisense (LICA) technology platform. It functions by reducing the production of PCSK9 in the liver, thus lowering the levels of plasma LDL-C (low-density lipoprotein cholesterol) and consequently decreasing the risk of cardiovascular diseases.
In April 2022, AstraZeneca released phase 2b clinical trial data for the subcutaneous injection of AZD8233 in treating hypercholesterolemia. The results demonstrated that a once-monthly dose of AZD8233 reduced patients' PCSK9 levels by 89% and LDL-C levels by 73%, achieving the primary endpoint of the trial. AstraZeneca indicated that this candidate drug achieved a greater reduction compared to the key trials for Praluent (alirocumab) from Sanofi/Regeneron, where alirocumab lowered LDL-C by approximately 50%.
How to obtain the latest development progress of PCSK9 inhibitors?
In the Synapse database, you can keep abreast of the latest research and development advances of PCSK9 inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!