FDA and EMA acknowledge submission for CSL's innovative Factor XIIa-blocking agent, Garadacimab
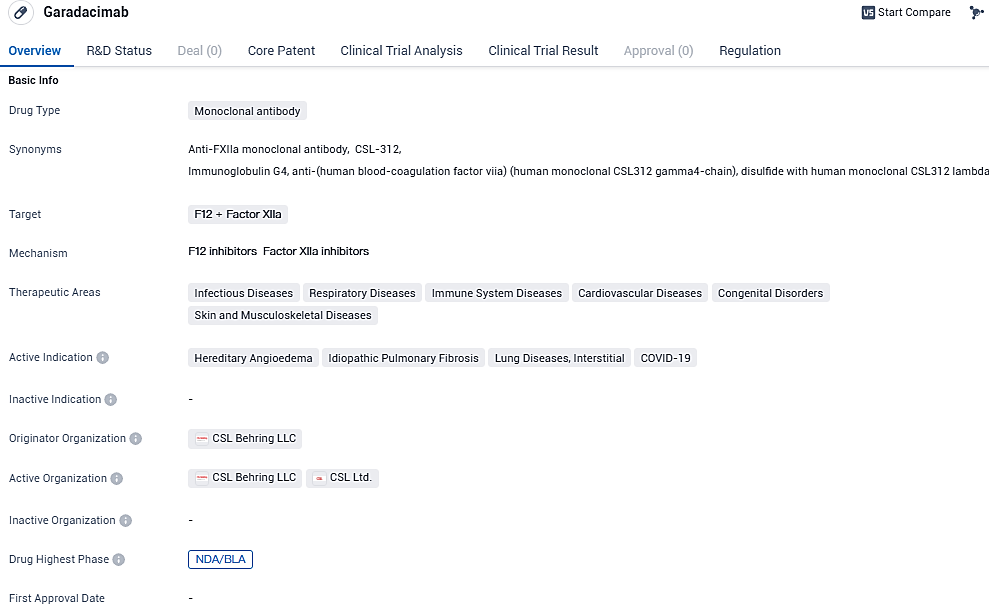
International biotech heavyweight CSL has disclosed that its Biologics License Application for the drug garadacimab (CSL312), intended as a once-a-month preventative measure for hereditary angioedema, has been officially received by the U.S. Food and Drug Administration.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
CSL has disclosed that the European Medicines Agency has commenced the review of its Marketing Authorization Application concerning garadacimab. Should it receive the nod of approval, garadacimab is slated to be the inaugural therapy targeting activated Factor XII (FXIIa) for HAE patients in both the U.S. and the European Union.
As an innovative therapy, garadacimab stands out as a pioneering recombinant monoclonal antibody fashioned to engage activated FXII. The role of FXIIa involves catalyzing the commencement of the kallikrein-kinin process, which is responsible for the HAE flare-ups. Garadacimab's approach of neutralizing FXIIa is different because it intervenes early in this cascade, setting it apart from other HAE treatments that work on alleviating symptoms through interaction with mediators found further down the chain.
Emmanuelle Lecomte Brisset, Pharm D, the Senior Vice President and the head honcho of Global Regulatory Affairs at CSL, expressed immense pride in terms of the legacy CSL has in concocting groundbreaking solutions for rare diseases. Lecomte Brisset highlighted the significance of garadacimab's advancement as the first internally developed recombinant monoclonal antibody as a testament to CSL's pledge to aid those grappling with HAE.
The acknowledgment of garadacimab as a potential orphan drug for treating hereditary angioedema has been given by both the FDA in the United States and the EMA in the European Union. The submissions for both the Biologics License Application and the Marketing Authorization Application draw from the cornerstone VANGUARD study. This critical trial was multi-centered, randomized, double-blind, and featured parallel groups. It primarily explored garadacimab's role and reliability as a preventative measure for HAE individuals. The detailed outcomes from the VANGUARD research have been documented in the prestigious medical journal, The Lancet.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
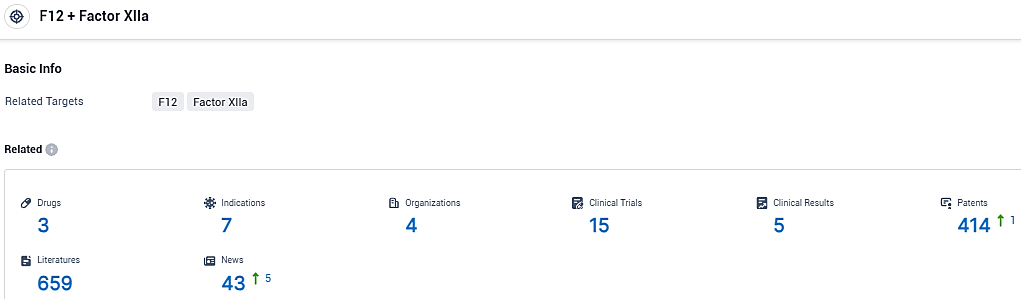
According to the data provided by the Synapse Database, As of December 27, 2023, there are 3 investigational drugs for the F12 and Factor XIIa target, including 7 indications, 4 R&D institutions involved, with related clinical trials reaching 15, and as many as 414 patents.
Garadacimab shows promising potential in the field of biomedicine. Its broad therapeutic areas and active indications indicate its versatility in treating various diseases. With its highest phase of development and orphan drug status, Garadacimab is poised to make a significant impact in the pharmaceutical industry and potentially improve the lives of patients suffering from these conditions.