An analysis of LY-3537982's R&D progress and its clinical results presented at the 2023 AACR Annual Meeting
Mutations in KRAS are among the most frequent oncogenic drivers with the G12C mutation found in up to ~13% of NSCLC. LY3537982 is an oral, highly selective, and potent inhibitor of KRAS G12C, which preclinically delivers >90% sustained target occupancy. The initial results from LOXO-RAS-20001, a phase 1 study of LY3537982 in patients (pts) with KRAS G12C-mutant advanced solid tumors were reported at the AACR Congress.
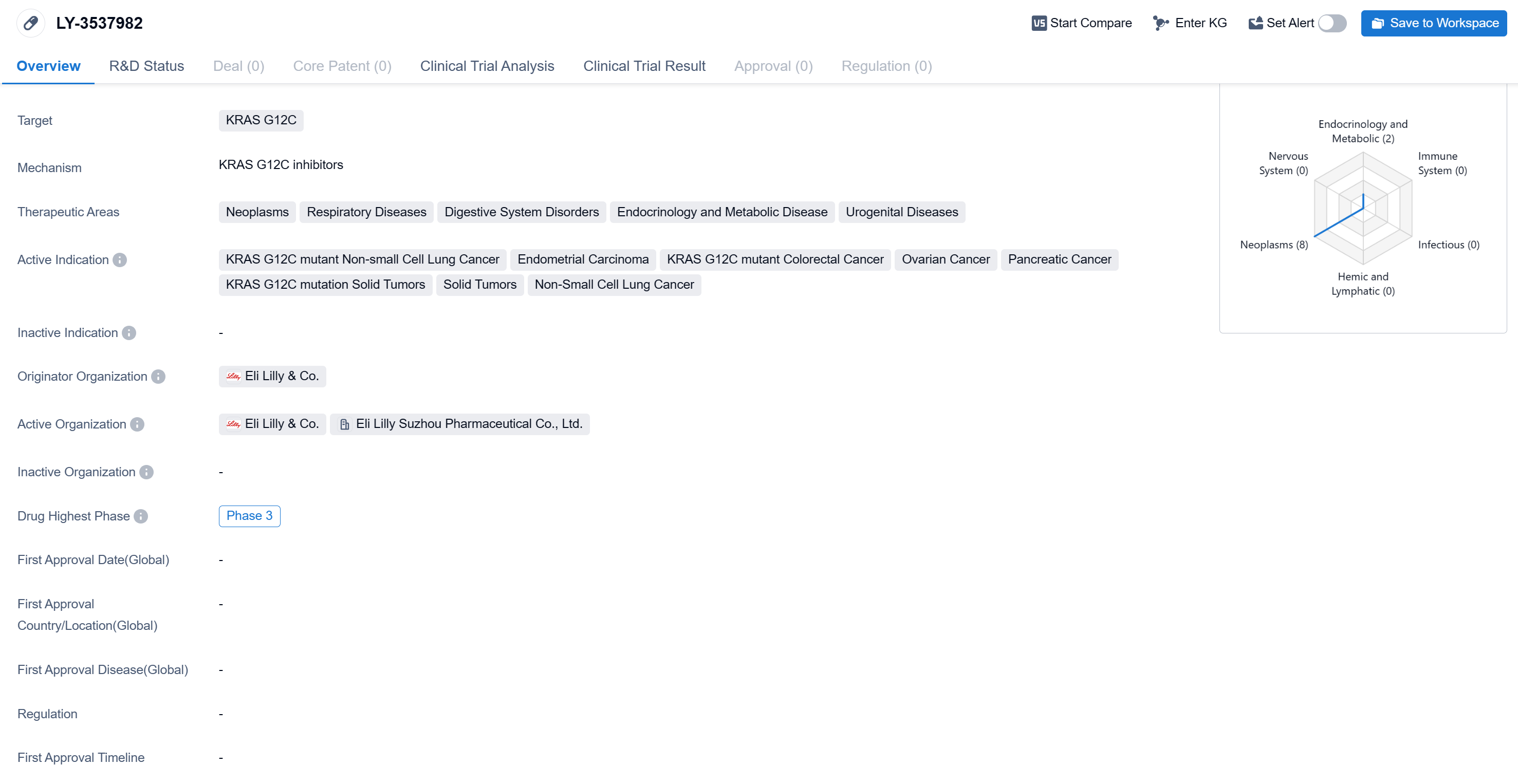
LY-3537982's R&D Progress
LY-3537982 is a small molecule drug developed by Eli Lilly & Co. It is designed to target the KRAS G12C mutation, which is associated with various types of cancers. The therapeutic areas that LY-3537982 aims to address include neoplasms (abnormal growth of cells), respiratory diseases, digestive system disorders, endocrinology and metabolic diseases, and urogenital diseases.
According to the Patsnap Synapse, LY-3537982 is currently in Phase 3 clinical trials globally. And the clinical trial distributions for LY-3537982 are primarily in the United States, China, and United Kingdom. The key indication is Neoplasm Metastasis. 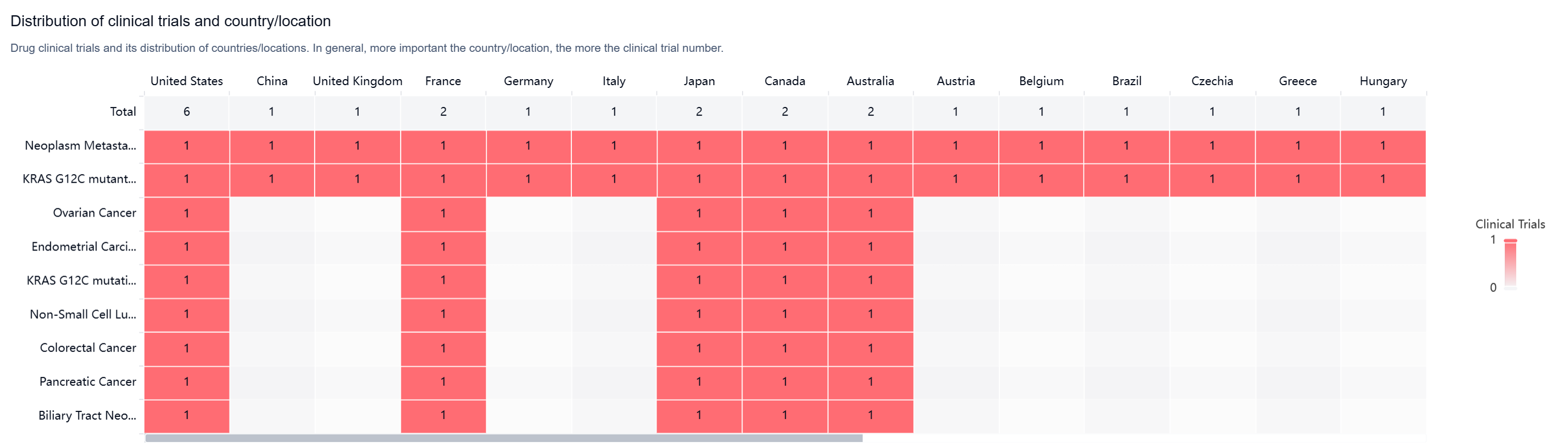
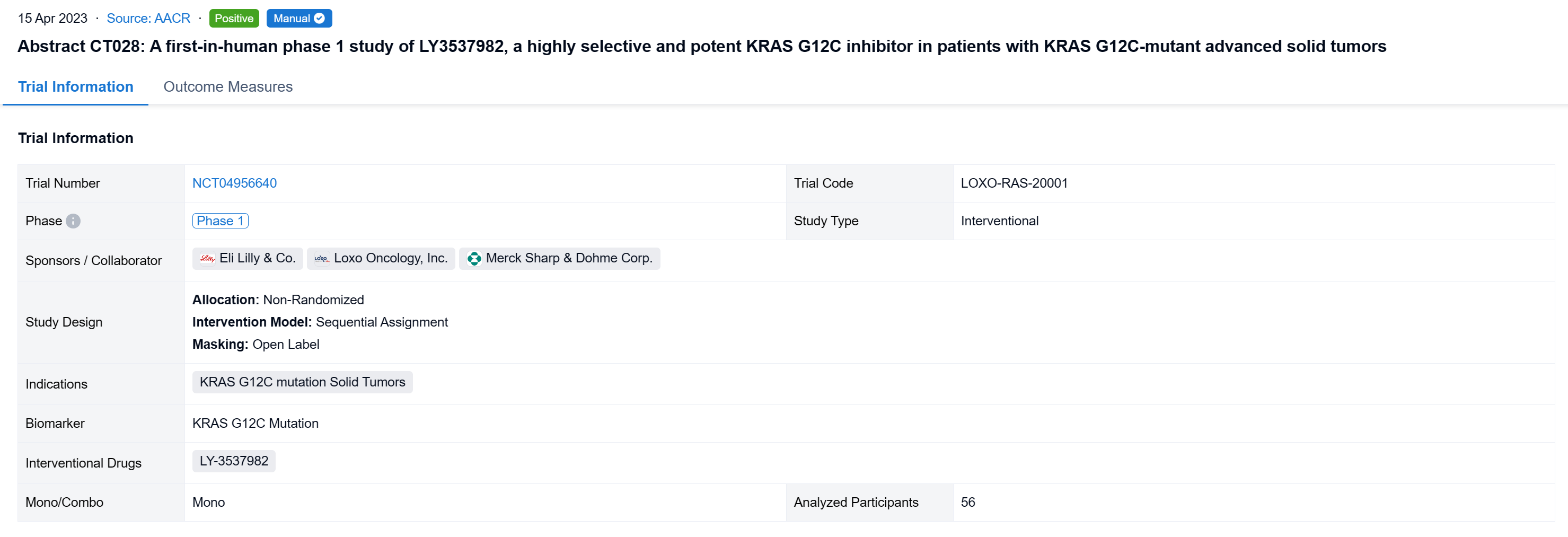
Detailed Clinical Result of LY-3537982
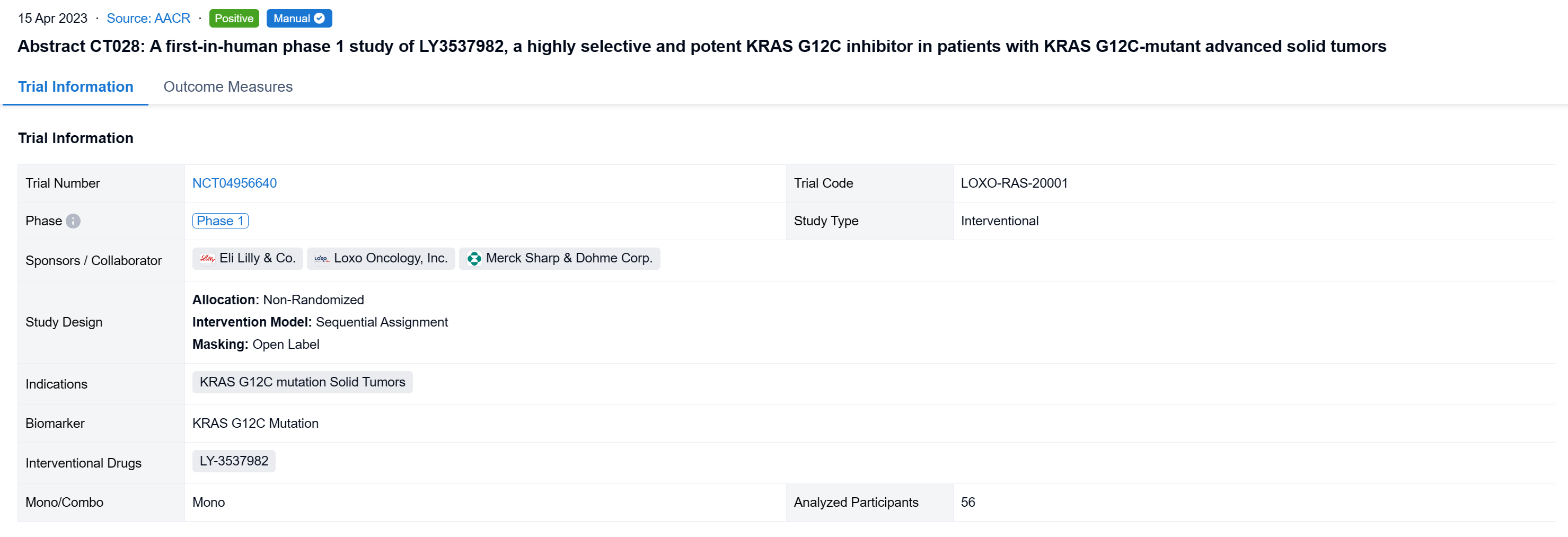
The non-randomized, sequential assignment, open-labeled clinical trial (NCT04956640) was aimed to evaluate the efficacy and safety of LY3537982 in patients (pts) with KRAS G12C-mutant advanced solid tumors.
In this study, LY3537982 monotherapy dose escalation followed a mTPI-2 method. Dose expansion cohorts included combinations with pembrolizumab (NSCLC) and cetuximab (CRC). Key objectives were to determine the RP2D of LY3537982, safety, PK, and antitumor activity per RECIST v1.1.
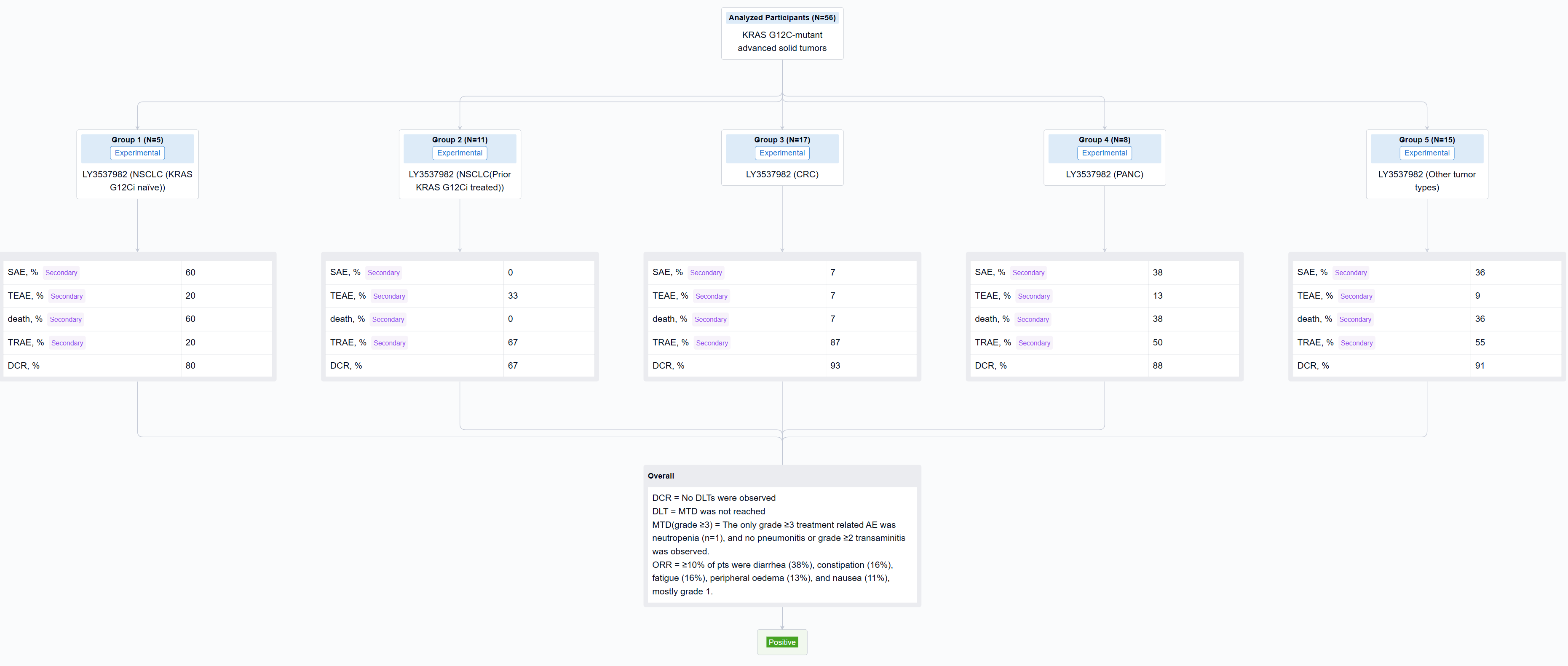
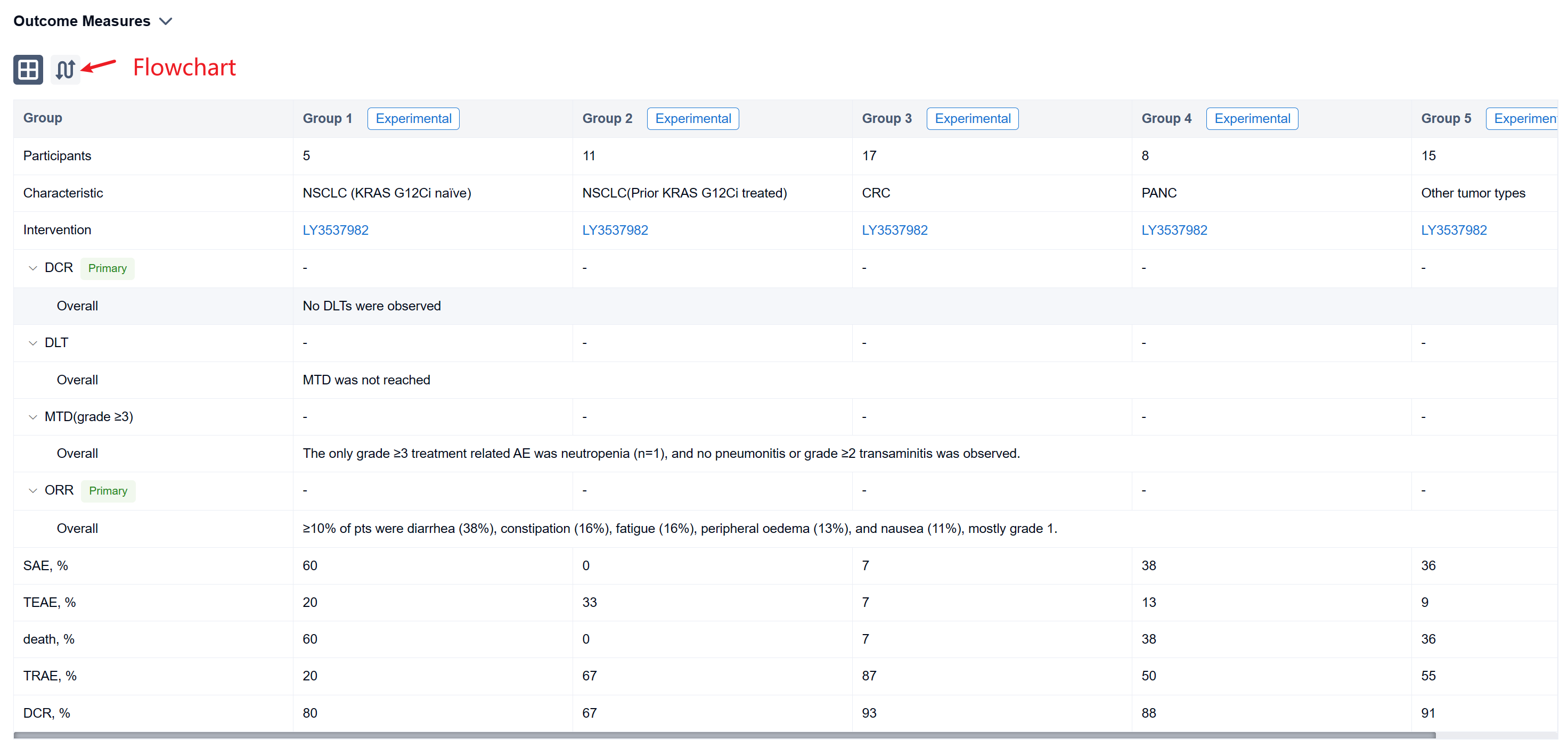
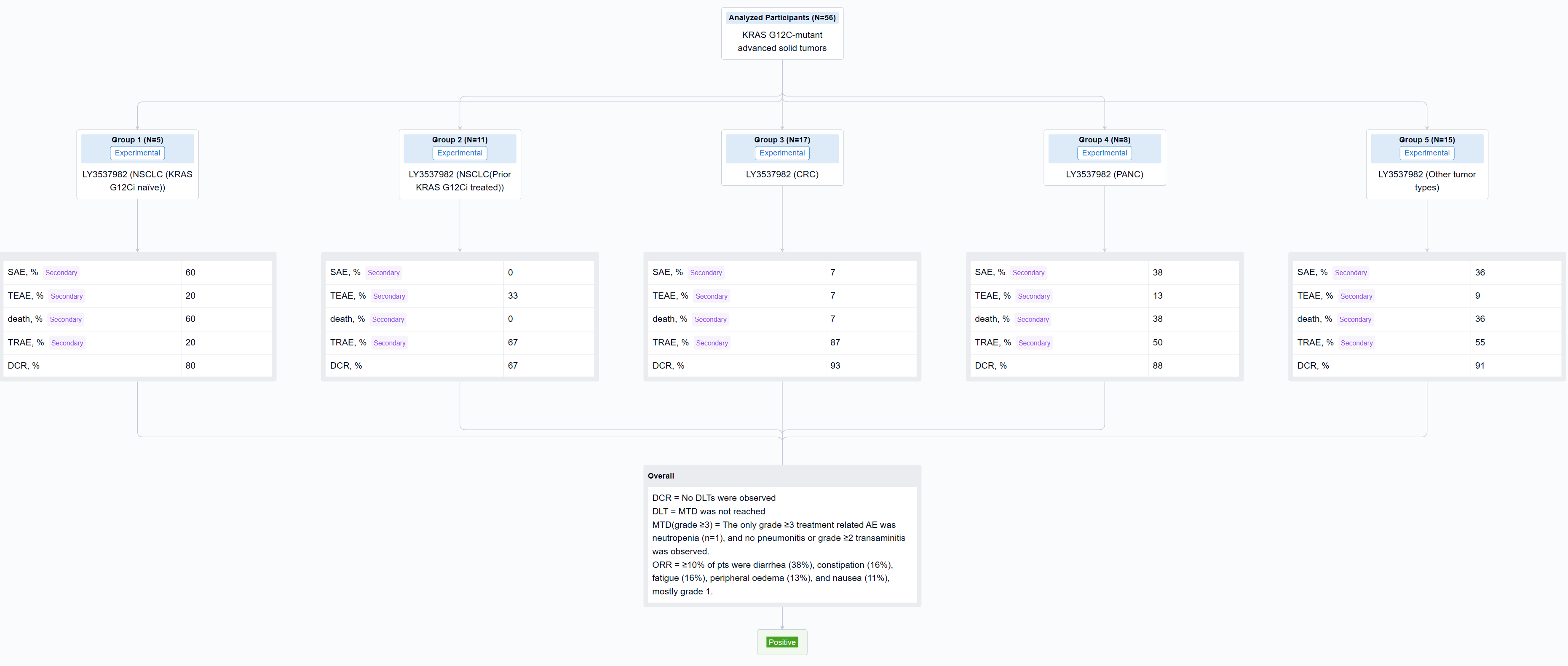
The result showed that As of 17 August 2022, 56 pts with NSCLC (16), CRC (17), PANC (8), and other tumor types (15) were treated with LY3537982 monotherapy on doses from 50-200 mg BID. Median number of prior systemic therapies was 2 (range, 0-8). No DLTs were observed, MTD was not reached, and RP2D determination is ongoing. Median time on treatment was 3 months (range, 0.3-13), 33 pts are ongoing, and 23 pts discontinued (none due to a related AE). TEAEs observed in ≥10% of pts were diarrhea (38%), constipation (16%), fatigue (16%), peripheral oedema (13%), and nausea (11%), mostly grade 1. The only grade ≥3 treatment related AE was neutropenia (n=1), and no pneumonitis or grade ≥2 transaminitis was observed. There were no treatment related serious AEs or deaths. Dose proportional steady-state exposures were observed through 150 mg BID. Table shows preliminary efficacy data.
It can be concluded that LY3537982 demonstrated a favorable safety profile, including the absence of high-grade liver toxicity, and tolerance in pts previously intolerant to other KRAS G12C inhibitors. Preliminary efficacy was observed with LY3537982 monotherapy across multiple tumor types. Updated data in more than 100 pts, including data in combination with pembrolizumab and cetuximab will be presented.
How to Easily View the Clinical Results Using Synapse Database?
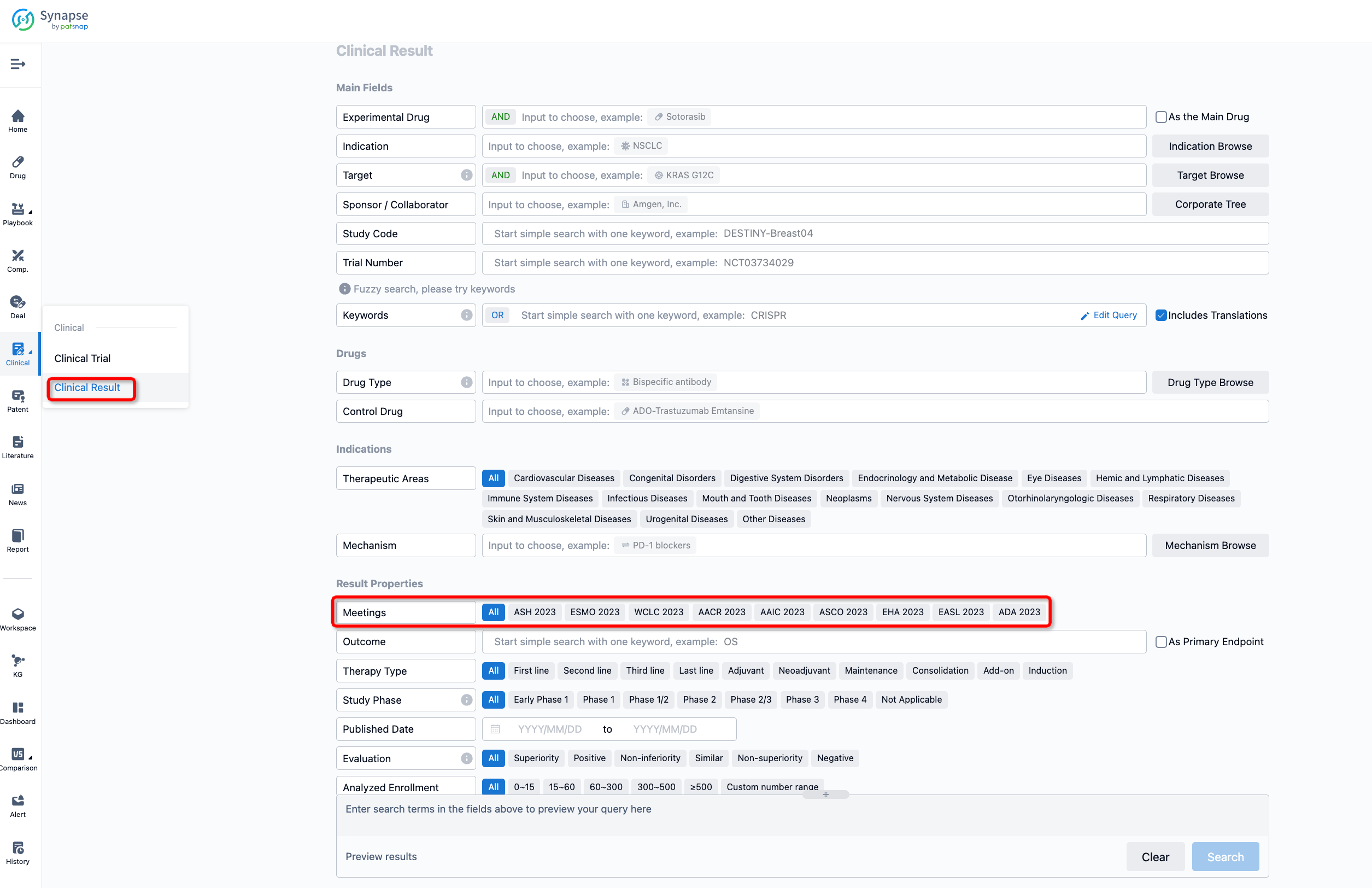
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
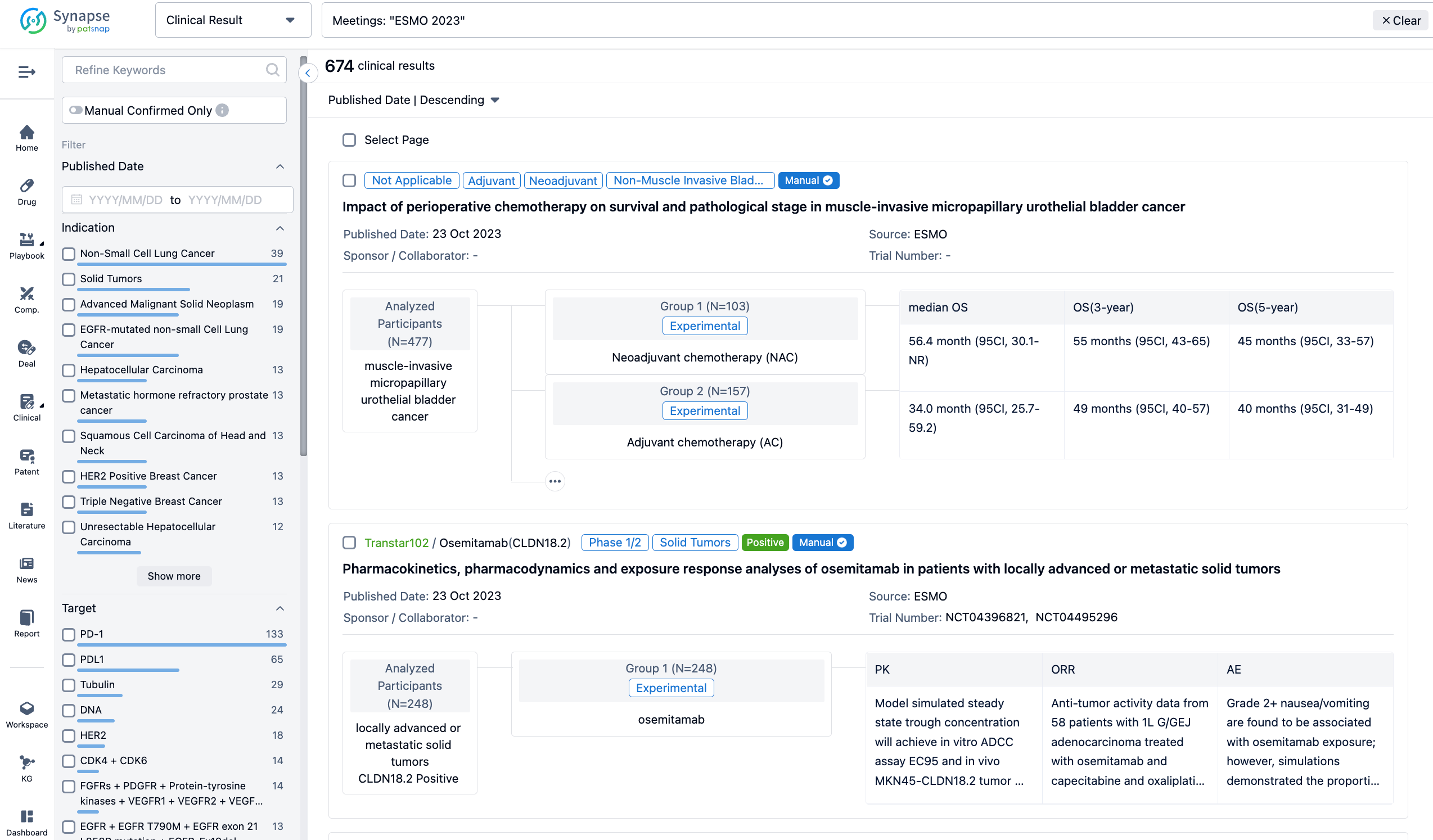
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!