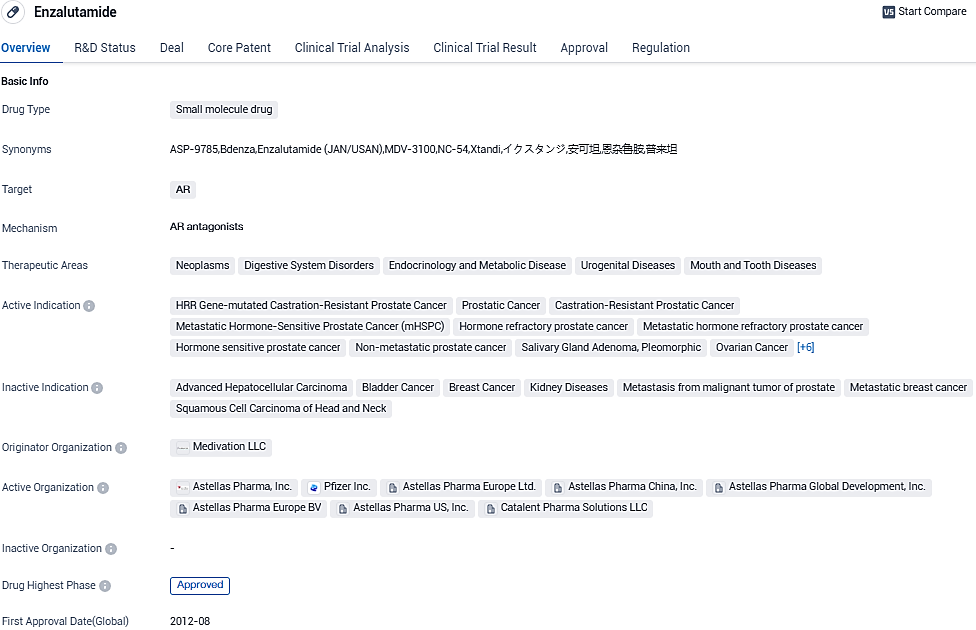
FDA approves XTANDI® for advanced prostate cancer treatment
Astellas Pharma Inc. along with Pfizer Inc. have declared that they have secured an endorsement from the FDA for a supplementary New Drug Application regarding XTANDI® (enzalutamide). This follows several sped-up development and scrutiny initiatives by the FDA including Priority Review designation, Fast Track designation, and Real-time Oncology Review, all of which were based on findings from the Phase 3 EMBARK study.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The endorsement of XTANDI, the exclusive androgen receptor signaling inhibitor licensed by the FDA, marks a landmark development in treatement of patients with nonmetastatic castration-sensitive prostate cancer that shows biochemical recurrence presenting increased risk for metastasis. Patients with nmCSPC who have high-risk BCR can receive XTANDI independently or together with a gonadotropin-releasing hormone analog therapy.
Rough estimates indicate that between 20-40% of men who have received definite treatments for prostate cancer, including radical prostatectomy, radiotherapy, or a combination, will exhibit biochemical recurrence within a decade. Nearly 90% of men suffering from high-risk BCR progress to metastatic disease, with nearly a third ending up in fatality due to their metastatic prostate cancer.
Courtney Bugler, the CEO of ZERO Prostate Cancer, reflected on the plight of these men who had prostate cancer, went into remission, but later was informed of a disease recurrence with potential for metastasis. The emotional burden is considerable. Courtney was positive about the approval of XTANDI, viewing it as an encouraging treatment choice that offers hope to patients and caregivers in these difficult times.
Chief Medical Officer of Strategic Innovation and Pharmacy, Dr. Neal Shore, MD, FACS, who has nearly four decades' worth of experience in treating prostate cancer patients, expressed the significance of this development. He stated, “The licensing of XTANDI for patients with nmCSPC with BCR exhibiting a high probability of metastasis is an important step forward. With enzalutamide, an androgen deprivation signaling inhibitor, now being part of the standard discussion for patient-physician decision-making."
XTANDI is presently being reviewed for approval by a number of other global health regulatory bodies, including the European Medicines Agency. The drug is being evaluated for a broader indication in nmHSPC with high-risk BCR based on outcomes from the EMBARK trial.
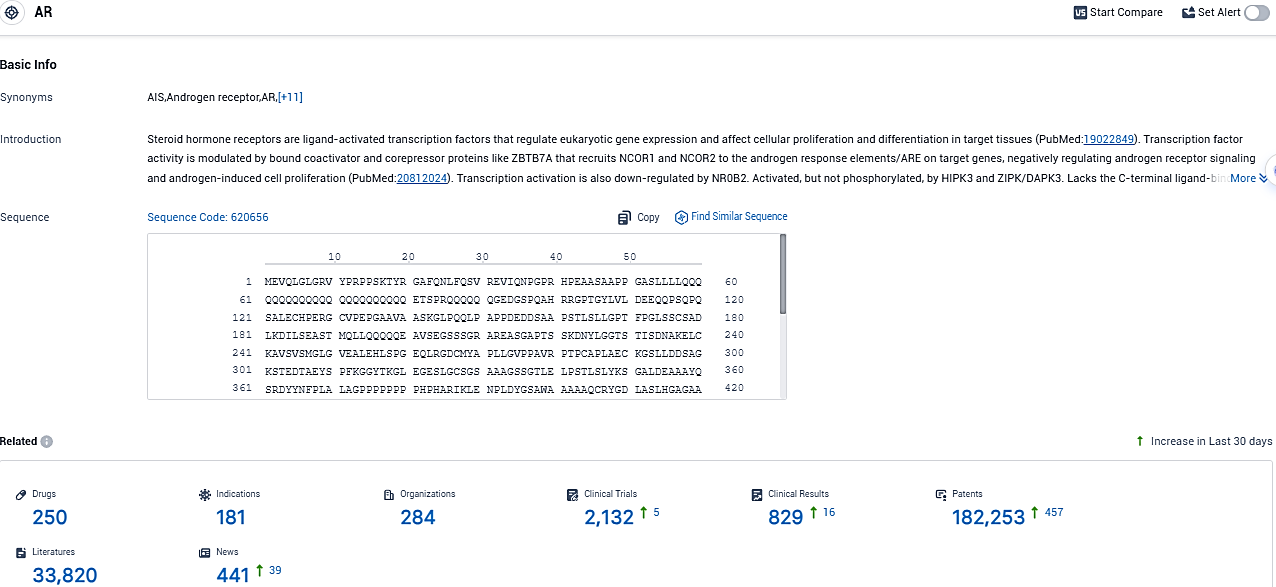
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 23, 2023, there are 250 investigational drugs for the AR target, including 181 indications, 284 R&D institutions involved, with related clinical trials reaching 2132, and as many as 182253 patents.
XTANDI® (enzalutamide) is an androgen receptor signaling inhibitor. XTANDI is currently approved for one or more of these indications in more than 90 countries, including in the U.S., European Union and Japan. Over one million patients have been treated with XTANDI globally.