An In-depth Analysis of acetaminophen/butalbital/caffeine's R&D Progress
Acetaminophen/butalbital/caffeine's R&D Progress
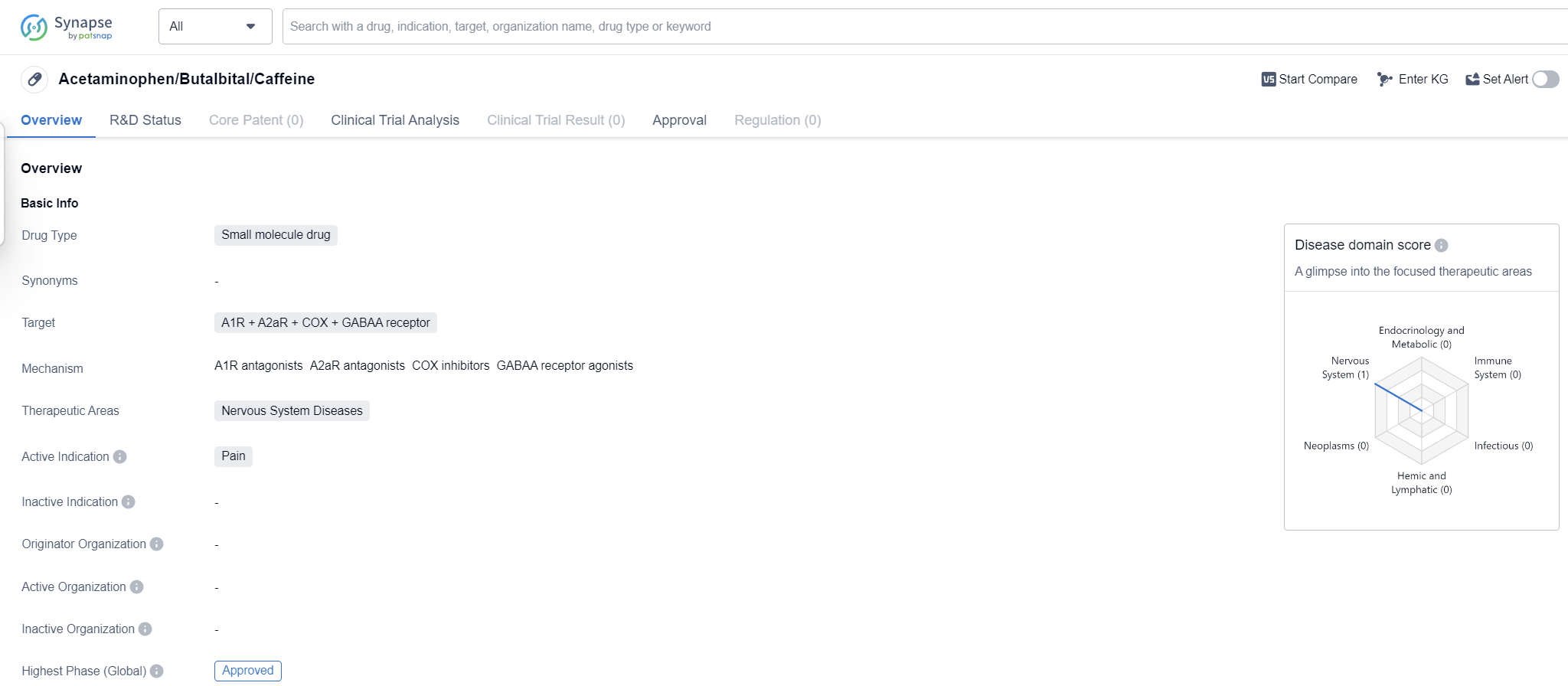
Acetaminophen/Butalbital/Caffeine is a small molecule drug that targets multiple receptors including A1R, A2aR, COX, and GABAA receptor. It falls under the therapeutic area of Nervous System Diseases and is primarily indicated for the treatment of pain.
The highest R&D phase of this drug is approved. This indicates that it has successfully completed all necessary clinical trials and has been granted regulatory approval for use in patients.
Acetaminophen, also known as paracetamol, is a widely used analgesic and antipyretic drug that is commonly used to relieve pain and reduce fever. It works by inhibiting the synthesis of prostaglandins, which are responsible for pain and inflammation. Butalbital is a barbiturate that acts as a central nervous system depressant and is used in combination with other drugs to treat tension headaches. Caffeine is a stimulant that can enhance the effects of other pain relievers and is often included in combination drugs for its ability to increase their efficacy.
The combination of acetaminophen, butalbital, and caffeine in this drug suggests a synergistic effect in relieving pain. Acetaminophen provides analgesic and antipyretic properties, butalbital acts as a muscle relaxant and sedative, and caffeine enhances the overall pain-relieving effects. By targeting multiple receptors including A1R, A2aR, COX, and GABAA receptor, the drug may provide a broader spectrum of pain relief compared to drugs that target a single receptor.
The approval of this drug indicates that it has undergone rigorous testing and has demonstrated safety and efficacy in treating pain. It may be prescribed for various types of pain, including tension headaches, migraines, and musculoskeletal pain. However, further information regarding specific indications, dosing, and potential side effects would be necessary to fully evaluate the drug's potential in clinical practice.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for acetaminophen/butalbital/caffeine: A1R antagonists A2aR antagonists COX inhibitors GABAA receptor agonists
A1R antagonists are drugs that block the A1 adenosine receptor. Adenosine receptors are a type of cell surface receptor that bind to the signaling molecule adenosine. A1 adenosine receptors are found in various tissues and organs throughout the body, including the brain, heart, and immune cells. By blocking the A1 adenosine receptor, A1R antagonists can modulate the effects of adenosine, which can have various physiological and pharmacological effects.
A2aR antagonists are drugs that block the A2a adenosine receptor. A2a adenosine receptors are predominantly found in the brain, but they are also present in other tissues such as the heart, lungs, and immune cells. By blocking the A2a adenosine receptor, A2aR antagonists can affect neurotransmission, cardiovascular function, and immune responses.
COX inhibitors, also known as cyclooxygenase inhibitors, are drugs that inhibit the activity of the enzyme cyclooxygenase. Cyclooxygenase is an enzyme involved in the production of prostaglandins, which are lipid signaling molecules that play a role in inflammation, pain, and fever. By inhibiting cyclooxygenase, COX inhibitors can reduce the production of prostaglandins and thereby alleviate inflammation, pain, and fever.
GABAA receptor agonists are drugs that activate the GABAA receptor. The GABAA receptor is a type of receptor found in the central nervous system that binds to the neurotransmitter gamma-aminobutyric acid (GABA). Activation of the GABAA receptor leads to inhibitory effects on neuronal activity, resulting in sedative, anxiolytic, and muscle relaxant effects. GABAA receptor agonists are commonly used as medications for anxiety, insomnia, epilepsy, and anesthesia.
In summary, A1R antagonists and A2aR antagonists modulate the effects of adenosine, COX inhibitors reduce inflammation, and GABAA receptor agonists have sedative and anxiolytic effects.
Drug Target R&D Trends for acetaminophen/butalbital/caffeine
The A1R (adenosine A1 receptor), A2aR (adenosine A2a receptor), COX (cyclooxygenase), and GABAA (gamma-aminobutyric acid type A) receptors play crucial roles in the human body. A1R and A2aR are adenosine receptors involved in regulating neurotransmission, cardiovascular function, and inflammation. COX enzymes are responsible for the synthesis of prostaglandins, which are involved in pain, fever, and inflammation. GABAA receptors are inhibitory neurotransmitter receptors that regulate neuronal excitability and play a role in anxiety, sedation, and epilepsy. Understanding the functions and interactions of these receptors is essential for developing targeted pharmaceutical interventions in various therapeutic areas.
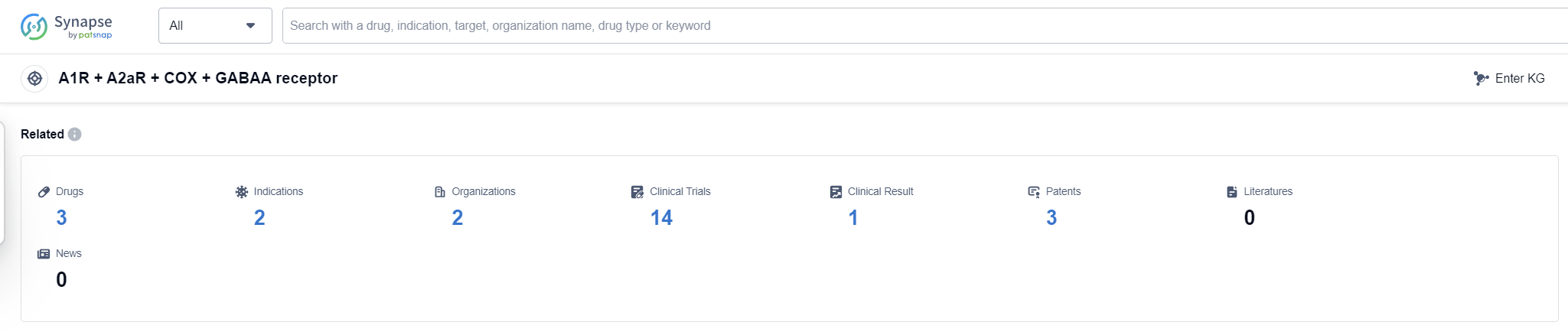
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 3 A1R + A2aR + COX + GABAA receptor drugs worldwide, from 2 organizations, covering 2 indications, and conducting 14 clinical trials
The analysis of the target A1R + A2aR + COX + GABAA receptor reveals that AbbVie, Inc. is the company with the highest phase of development, indicating their strong focus on this target. Drugs targeting this receptor have been approved for the indications of Tension-Type Headache and Pain. Small molecule drugs are progressing rapidly, suggesting intense competition in this area. The United States is leading in the development of drugs targeting this receptor. Overall, the current competitive landscape for the target A1R + A2aR + COX + GABAA receptor shows promising R&D progress, particularly in the areas of company focus, approved indications, drug types, and country/location development.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Acetaminophen/Butalbital/Caffeine is a small molecule drug that targets multiple receptors and is indicated for the treatment of pain. It has reached the highest phase of development, indicating regulatory approval for use. The combination of acetaminophen, butalbital, and caffeine suggests a synergistic effect in relieving pain, and the drug may offer a broader spectrum of pain relief compared to single-target drugs. Further information would be required to fully assess its clinical potential.