An In-depth Analysis of Brentuximab Vedotin's R&D Progress and Mechanism of Action on Drug Target
Brentuximab Vedotin's R&D Progress
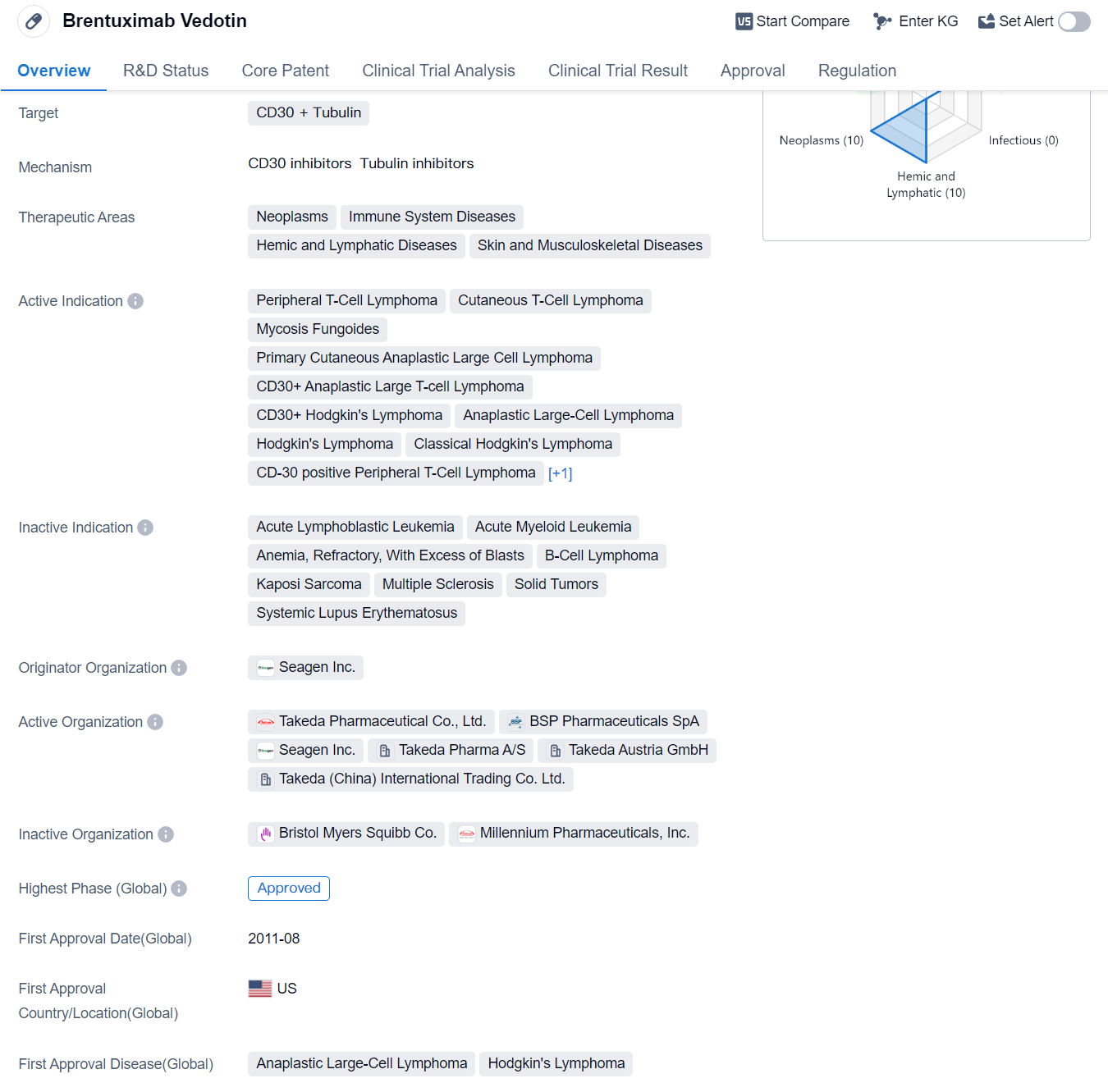
Brentuximab Vedotin is a drug classified as a monoclonal antibody and an antibody drug conjugate (ADC). It targets CD30 and Tubulin, making it effective in treating various diseases related to the immune system, neoplasms, hemic and lymphatic diseases, as well as skin and musculoskeletal diseases.
The drug has been approved for several indications, including peripheral T-cell lymphoma, cutaneous T-cell lymphoma, mycosis fungoides, primary cutaneous anaplastic large cell lymphoma, CD30+ anaplastic large T-cell lymphoma, CD30+ Hodgkin's lymphoma, anaplastic large-cell lymphoma, classical Hodgkin's lymphoma, CD-30 positive peripheral T-cell lymphoma, and systemic scleroderma.
Brentuximab Vedotin was first approved in the United States in August 2011 and has since received approval in other countries. It is developed by Seagen Inc., a pharmaceutical company specializing in the development of targeted cancer therapies.
The drug has reached the highest phase of development which is approved globally. It has undergone priority review, accelerated approval, and has been designated as an orphan drug and breakthrough therapy. These regulatory designations highlight the drug's potential to address unmet medical needs and expedite its development and approval process.
Brentuximab Vedotin's mechanism of action involves targeting CD30, a protein found on the surface of certain cells, and tubulin, a protein involved in cell division. By binding to CD30 and tubulin, the drug delivers a potent anti-cancer agent directly to the cancer cells, leading to their destruction.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Brentuximab Vedotin: CD30 inhibitor and Tubulin inhibitor
CD30 inhibitors are a type of medication that target and inhibit the activity of CD30, a protein found on the surface of certain cells. CD30 is primarily expressed on activated immune cells, such as T cells and B cells, as well as on some cancer cells. By inhibiting CD30, these inhibitors can interfere with the growth and survival of cancer cells that overexpress CD30, making them a potential treatment option for certain types of cancer, such as Hodgkin lymphoma and anaplastic large cell lymphoma.
Tubulin inhibitors, on the other hand, are a class of drugs that target tubulin, a protein involved in the formation of microtubules, which are essential for cell division. These inhibitors can disrupt the assembly and disassembly of microtubules, leading to cell cycle arrest and ultimately cell death. They are commonly used in cancer chemotherapy to prevent the proliferation of rapidly dividing cancer cells.
In summary, CD30 inhibitors specifically target the CD30 protein to inhibit cancer cell growth, while tubulin inhibitors disrupt the function of tubulin to prevent cell division in cancer cells.
Drug Target R&D Trends for Brentuximab Vedotin
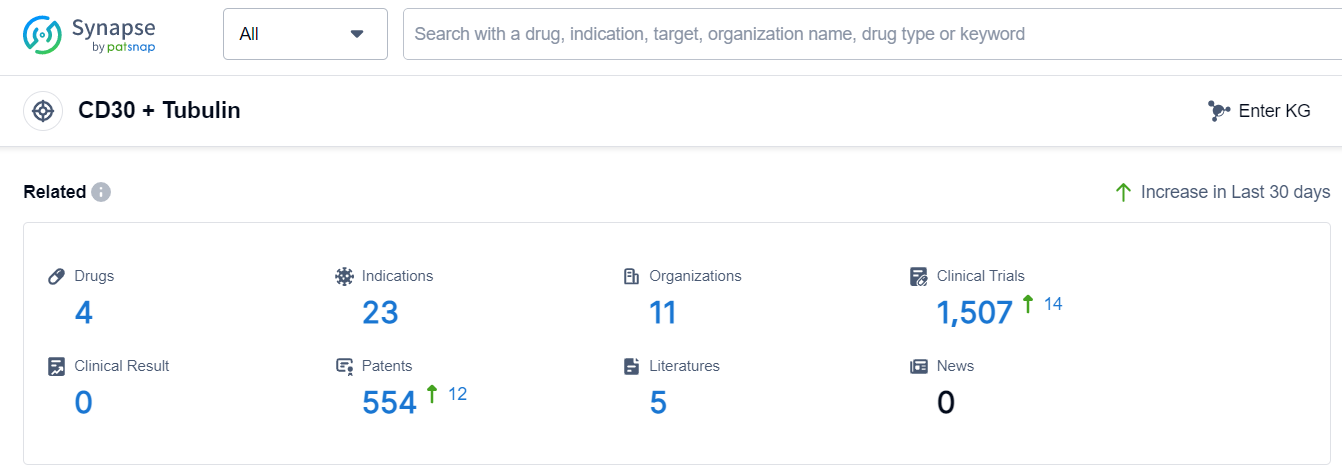
The analysis of the current competitive landscape of target CD30 + Tubulin reveals that Seagen Inc. and Takeda Pharmaceutical Co., Ltd. are the leading companies with drugs in advanced stages of development. The approved drugs under this target have shown efficacy in treating various lymphomas, including Hodgkin's Lymphoma and Anaplastic Large-Cell Lymphoma. Monoclonal antibodies and ADCs are the most rapidly progressing drug types, indicating intense competition in the market. The involvement of multiple countries, including China, highlights the global nature of research and development efforts. Overall, the future development of target CD30 + Tubulin holds promise for the treatment of lymphomas and related conditions.
According to Patsnap Synapse, as of 18 Sep 2023, there are a total of 4 CD30 + Tubulin drugs worldwide, from 11 organizations, covering 23 indications, and conducting 1507 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Brentuximab Vedotin is a monoclonal antibody and antibody drug conjugate that targets CD30 and tubulin. It has been approved for various indications related to neoplasms, immune system diseases, hemic and lymphatic diseases, and skin and musculoskeletal diseases. Developed by Seagen Inc., the drug has received global approvals and has undergone priority review, accelerated approval, and has been designated as an orphan drug and breakthrough therapy. Its approval in 2011 marked an important milestone in the treatment of the indicated diseases.