An In-depth Analysis of Etonogestrel's R&D Progress and Mechanism of Action on Drug Target
Etonogestrel's R&D Progress
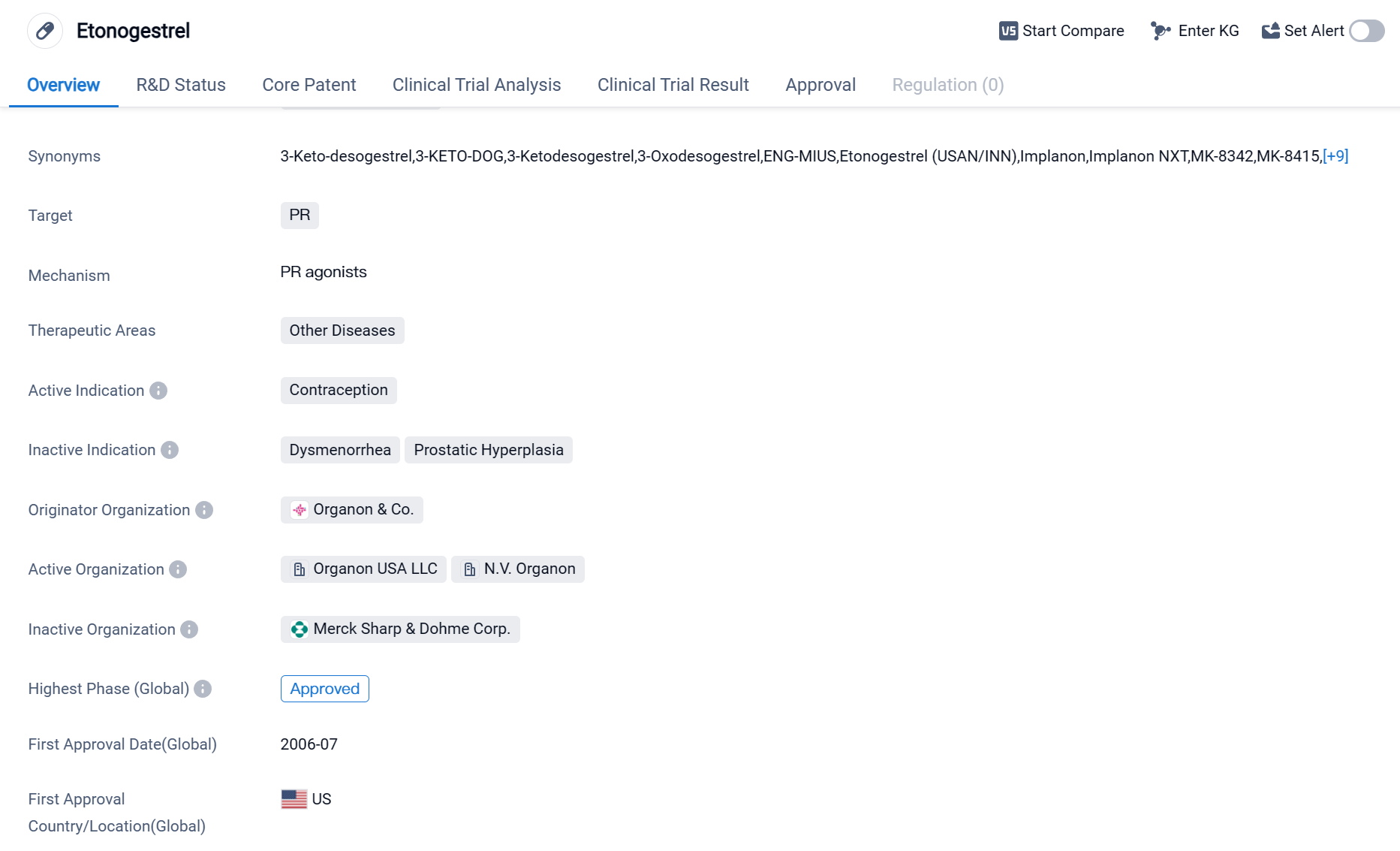
Etonogestrel is a small molecule drug primarily used for contraception. It targets the progesterone receptor (PR) and falls under the therapeutic area of other diseases. The drug was first approved in the United States in July 2006 by the originator organization, Organon & Co.
As a small molecule drug, Etonogestrel is designed to interact with specific receptors in the body to produce a desired therapeutic effect. In this case, it targets the progesterone receptor, which is involved in regulating the menstrual cycle and plays a crucial role in contraception. By binding to the PR, Etonogestrel prevents ovulation, thickens cervical mucus, and alters the lining of the uterus, making it less receptive to fertilized eggs.
The primary indication for Etonogestrel is contraception, making it a popular choice among women looking for reliable birth control methods. The approval of Etonogestrel in the United States in 2006 marked a significant milestone for the drug, as it became the first country to approve its use. This suggests that the United States recognized the importance of providing women with a reliable and effective contraceptive option.
Organon & Co. is the originator organization of Etonogestrel, implying that they were responsible for its initial development and subsequent regulatory approvals. The drug has reached the highest phase of approval globally, indicating its widespread acceptance and availability in these markets.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Etonogestrel: PR agonists
PR agonists are a type of medication that activate the progesterone receptor (PR) in the body. Progesterone is a hormone that plays a crucial role in the female reproductive system, particularly in regulating the menstrual cycle and supporting pregnancy. PR agonists mimic the effects of progesterone by binding to the PR and initiating a cascade of cellular responses. These agonists can be used in various medical applications, such as hormone replacement therapy, contraception, and treatment of certain gynecological conditions. By activating the PR, PR agonists can help regulate hormone levels and promote specific physiological processes.
Drug Target R&D Trends for Etonogestrel
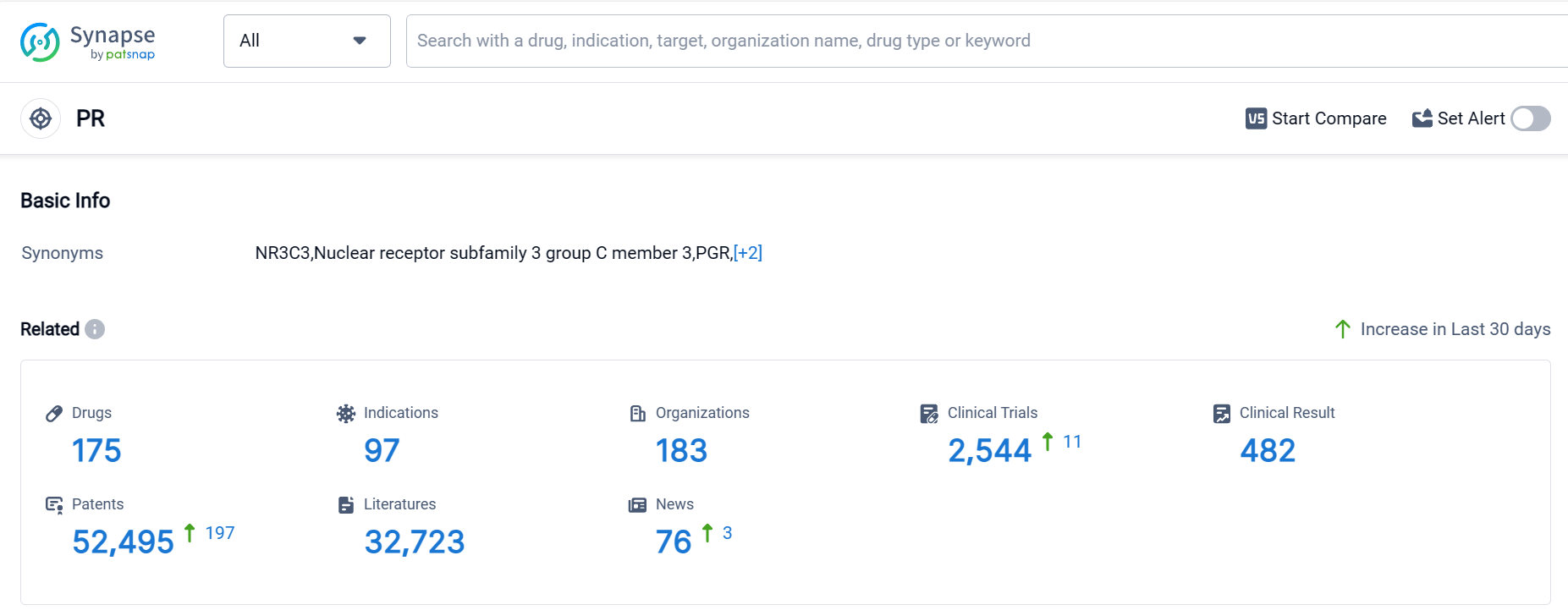
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 175 PR drugs worldwide, from 183 organizations, covering 97 indications, and conducting 2544 clinical trials.
The current competitive landscape for the target PR is characterized by the presence of several companies actively developing drugs. Bayer AG, Pfizer Inc., and ASKA Pharmaceutical Holdings Co., Ltd. are among the companies with the highest number of approved drugs and advanced stages of development. The focus of drug development is on indications such as contraception, pregnancy, osteoporosis (postmenopausal), and menstruation disturbances. Small molecule drugs are the most rapidly progressing drug type. The United States, China, and the European Union are the leading countries/locations in terms of drug development. China, in particular, has shown significant progress in developing drugs for the target PR. Overall, the target PR presents a competitive landscape with multiple companies and countries actively involved in research and development, indicating a promising future for the development of drugs in this area.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Etonogestrel is a small molecule drug developed by Organon & Co. that targets the progesterone receptor for contraception. It has been approved in the United States since 2006 and is widely available globally. Its mechanism of action involves preventing ovulation, thickening cervical mucus, and altering the uterine lining.