A Comprehensive Review of Lomustine's R&D Innovations and Drug Target Mechanism
Lomustine's R&D Progress
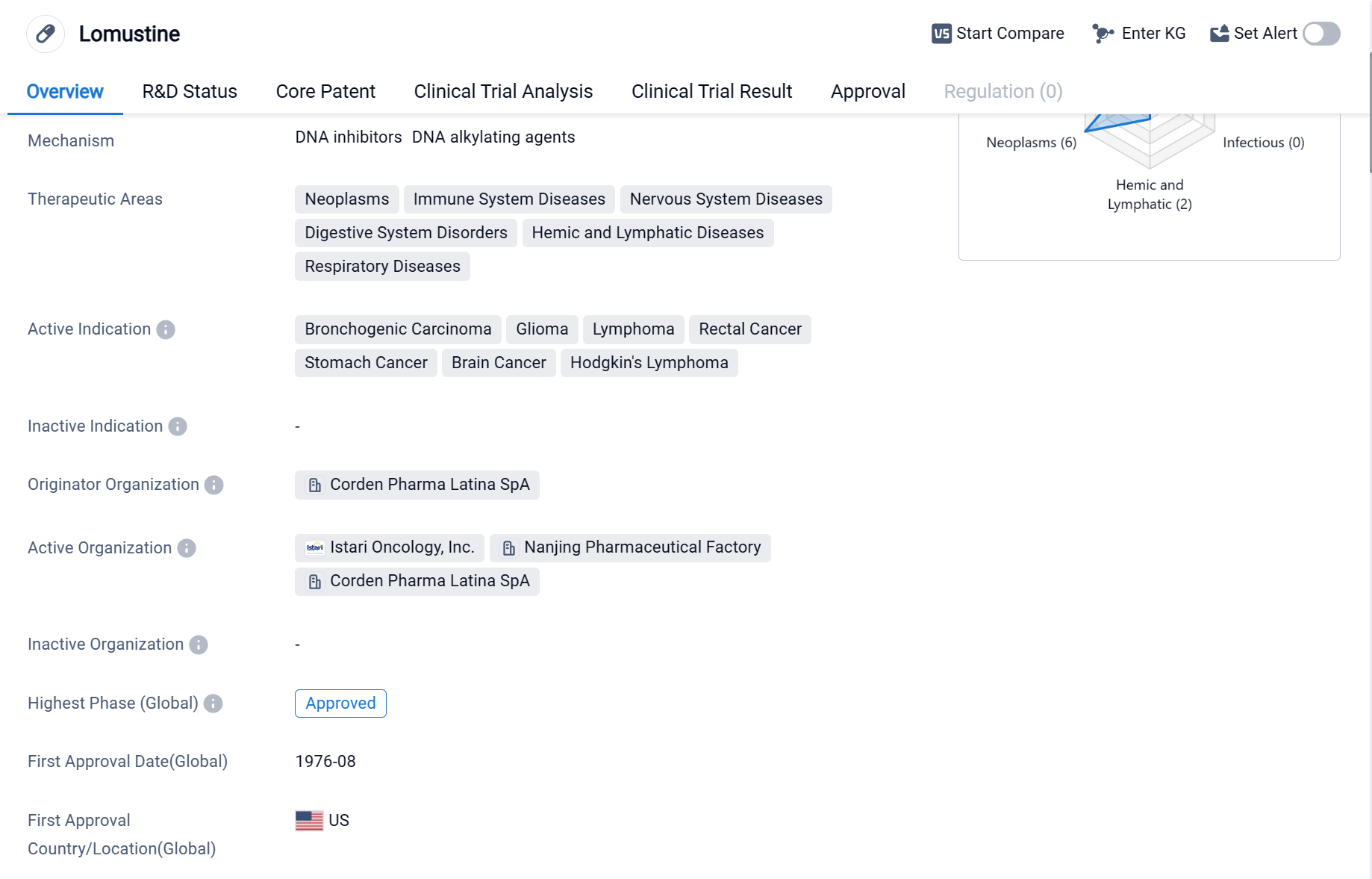
Lomustine is a small molecule drug that primarily targets DNA. It has been approved for use in various therapeutic areas, including neoplasms, immune system diseases, nervous system diseases, digestive system disorders, hemic and lymphatic diseases, and respiratory diseases. The drug has shown efficacy in treating bronchogenic carcinoma, glioma, lymphoma, rectal cancer, stomach cancer, brain cancer, and Hodgkin's lymphoma.
Lomustine was first approved in the United States in August 1976, making it a well-established drug in the pharmaceutical market. It is important to note that the drug has also received approval in China, indicating its global recognition and acceptance.
The originator organization of Lomustine is Corden Pharma Latina SpA, a pharmaceutical company known for its expertise in the development and production of various drugs. The highest R&D phase of this drug is approved.
Lomustine's mechanism of action involves targeting DNA, which plays a crucial role in the growth and replication of cancer cells. By interfering with DNA synthesis and repair, Lomustine inhibits the proliferation of cancer cells, ultimately leading to their death.
The drug's broad therapeutic areas and active indications highlight its potential in treating various types of cancers, including lung, brain, rectal, stomach, and lymphatic cancers. This versatility makes Lomustine a valuable option for physicians and patients seeking effective treatment options.
Given its long history of approval and widespread use, Lomustine has likely established a solid reputation in the medical community. Its approval in both the United States and China further strengthens its position as a reliable and globally recognized drug.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Lomustine: DNA inhibitors and DNA alkylating agents
DNA inhibitors are substances that interfere with the normal functioning of DNA, which is the genetic material present in all living organisms. These inhibitors can act by directly binding to DNA and preventing its replication or transcription, or by affecting enzymes involved in DNA synthesis or repair.
DNA alkylating agents are a specific type of DNA inhibitor that work by chemically modifying the DNA molecule. These agents contain alkyl groups that can covalently attach to the DNA, leading to the formation of abnormal DNA structures or crosslinks between DNA strands. This can disrupt the DNA helix and interfere with its ability to be replicated or transcribed, ultimately inhibiting cell division and causing cell death.
From a biomedical perspective, DNA inhibitors and DNA alkylating agents are important in the field of cancer treatment. Many cancer cells have uncontrolled growth and division, and targeting their DNA can help prevent further proliferation. DNA inhibitors, including DNA alkylating agents, are commonly used in chemotherapy to kill cancer cells or prevent their growth. These agents can be administered orally or intravenously, and their specific mechanisms of action can vary depending on the type of inhibitor used.
Drug Target R&D Trends for Lomustine
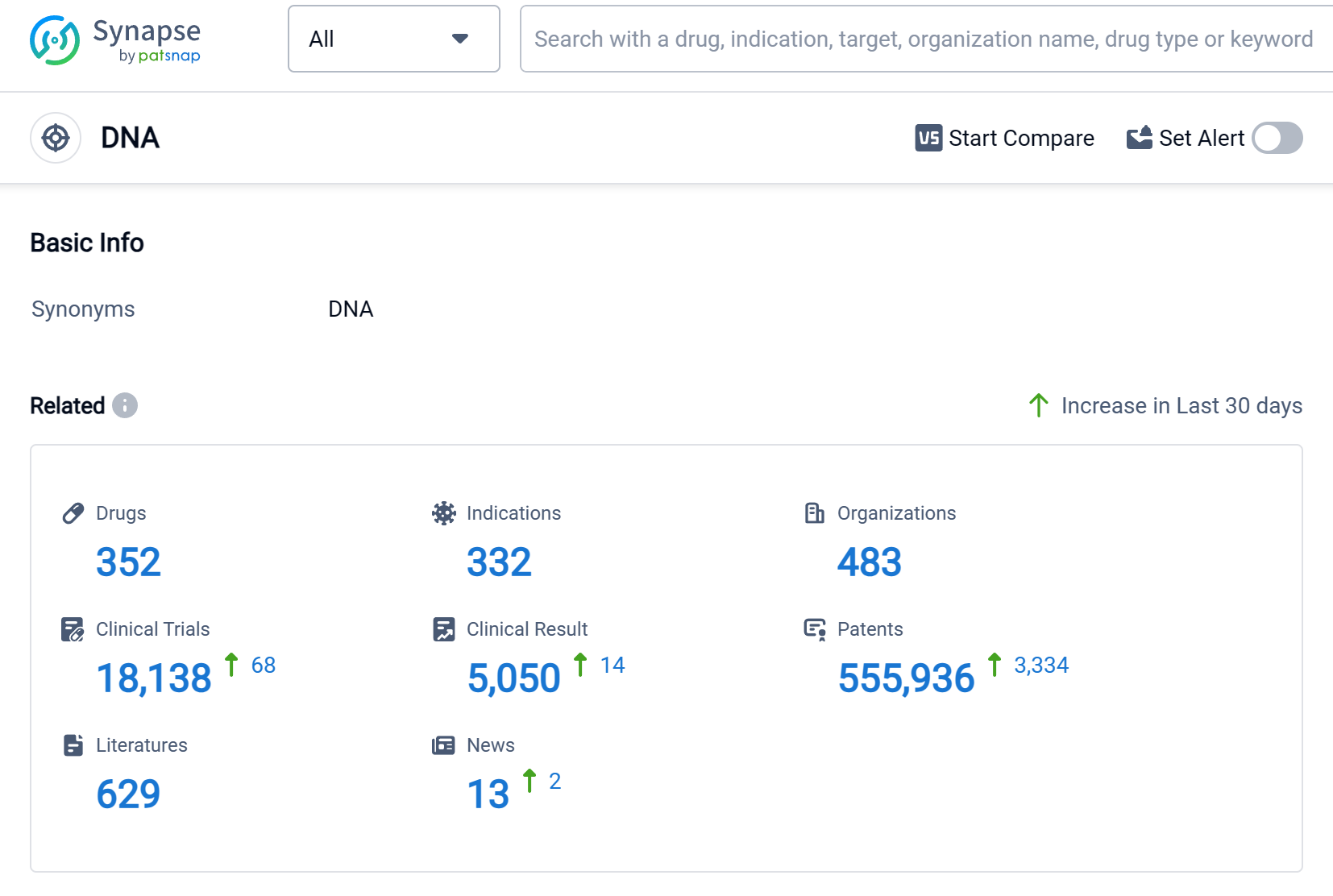
According to Patsnap Synapse, as of 2 Sep 2023, there are a total of 352 DNA drugs worldwide, from 483 organizations, covering 332 indications, and conducting 18138 clinical trials.
The analysis of the target DNA in the pharmaceutical industry reveals that Pfizer Inc., Novartis AG, Baxter International, Inc., Takeda Pharmaceutical Co., Ltd., and Johnson & Johnson are the companies growing fastest under this target. Lymphoma, Ovarian Cancer, and Breast Cancer are the indications with the highest number of approved drugs. Small molecule drugs, Monoclonal antibodies, and Antibody drug conjugates are the drug types progressing most rapidly. China, the United States, Japan, and the European Union are the countries/locations developing fastest under the target DNA. The current competitive landscape indicates intense competition, particularly in the biosimilar market. The future development of the target DNA holds potential for further advancements in drug development, particularly in China and other key countries/locations.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Lomustine is a small molecule drug developed by Corden Pharma Latina SpA. It targets DNA and has been approved for the treatment of various neoplasms, immune system diseases, nervous system diseases, digestive system disorders, hemic and lymphatic diseases, and respiratory diseases. With active indications in multiple types of cancers, Lomustine has proven its efficacy and is widely used in the medical field. Its approval in both the United States and China further demonstrates its global recognition and acceptance.