An In-depth Analysis of Lonafarnib's R&D Progress and Mechanism of Action on Drug Target
Lonafarnib's R&D Progress
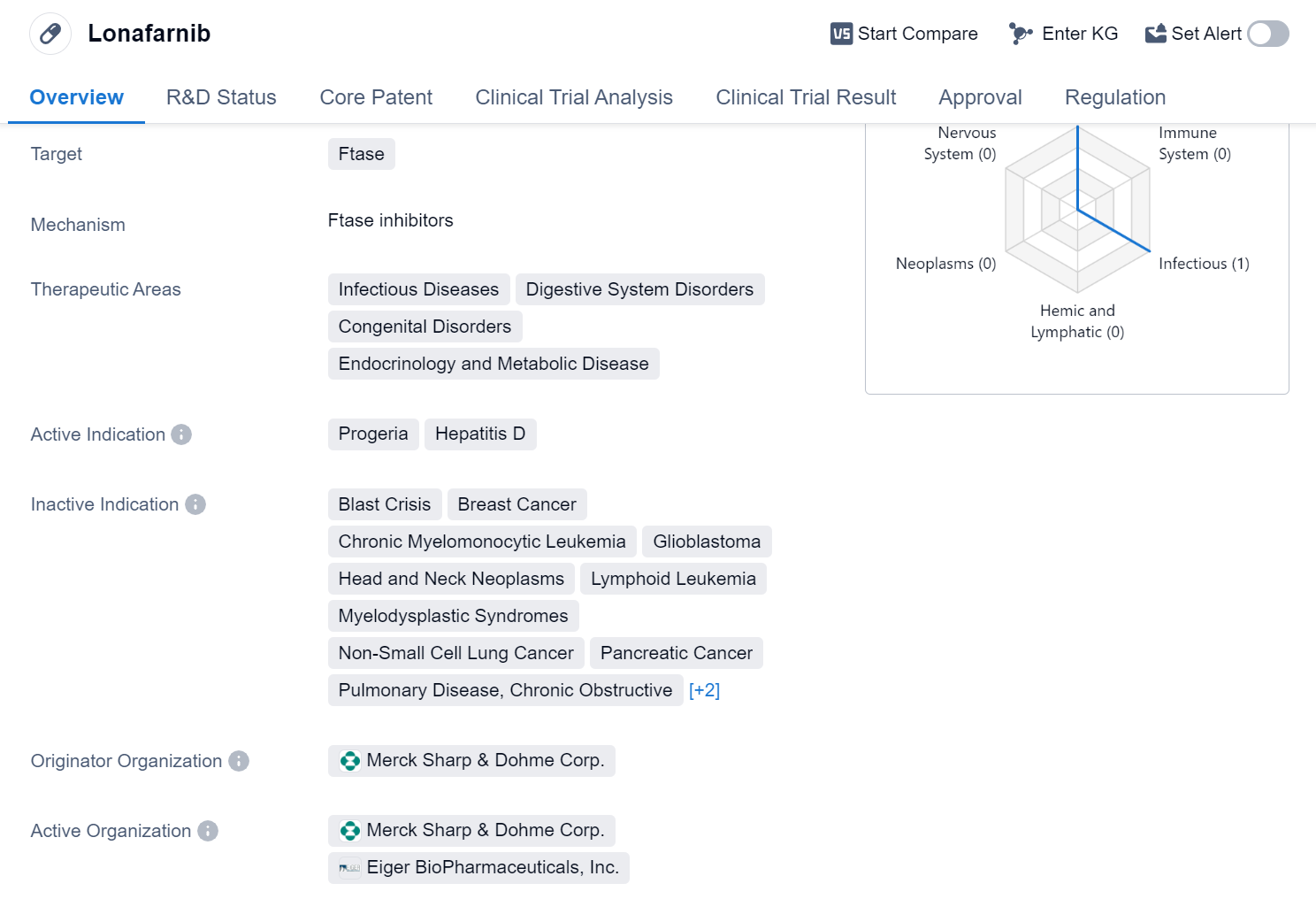
Lonafarnib is a small molecule drug that targets Ftase, an enzyme involved in protein prenylation. It has been developed by Merck Sharp & Dohme Corp. and has received approval for use in the treatment of certain conditions. Lonafarnib falls under the therapeutic areas of Infectious Diseases, Digestive System Disorders, Congenital Disorders, Endocrinology, and Metabolic Disease.
The drug has shown efficacy in treating Progeria, a rare genetic disorder that causes rapid aging in children. Lonafarnib has been approved for this indication in the United States, with its first approval date recorded in November 2020. The approval was granted based on its potential to address an unmet medical need in this specific patient population.
Lonafarnib has also been approved for the treatment of Hepatitis D, a viral infection that affects the liver. This approval further highlights the drug's potential in addressing infectious diseases. The breakthrough therapy designation and orphan drug status indicate that Lonafarnib has demonstrated promising results in clinical trials and is intended to treat rare diseases.
The approval of Lonafarnib in the United States signifies a significant milestone for Merck Sharp & Dohme Corp. and the field of biomedicine. It provides healthcare professionals with a new treatment option for Progeria and Hepatitis D patients, potentially improving their quality of life and prognosis.
As a small molecule drug, Lonafarnib offers advantages such as ease of manufacturing, oral administration, and potential for targeted delivery. These characteristics make it a valuable addition to the pharmaceutical industry's arsenal against various diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Lonafarnib: Ftase inhibitors
Ftase inhibitors are a type of drug that target the enzyme farnesyltransferase (Ftase). Ftase is responsible for adding a farnesyl group to proteins, a process known as protein farnesylation. This modification is important for the proper functioning of certain proteins involved in cell signaling and growth.
By inhibiting Ftase, these inhibitors prevent the farnesylation of proteins, which can disrupt the signaling pathways that contribute to the growth and survival of cancer cells. Therefore, Ftase inhibitors have been investigated as potential anti-cancer agents.
From a biomedical perspective, Ftase inhibitors are a promising class of drugs that have shown potential in cancer therapy. They work by specifically targeting the Ftase enzyme and interfering with the farnesylation process, which is crucial for the growth and proliferation of cancer cells. By inhibiting this process, Ftase inhibitors can potentially inhibit the growth of tumors and improve patient outcomes.
Drug Target R&D Trends for Lonafarnib
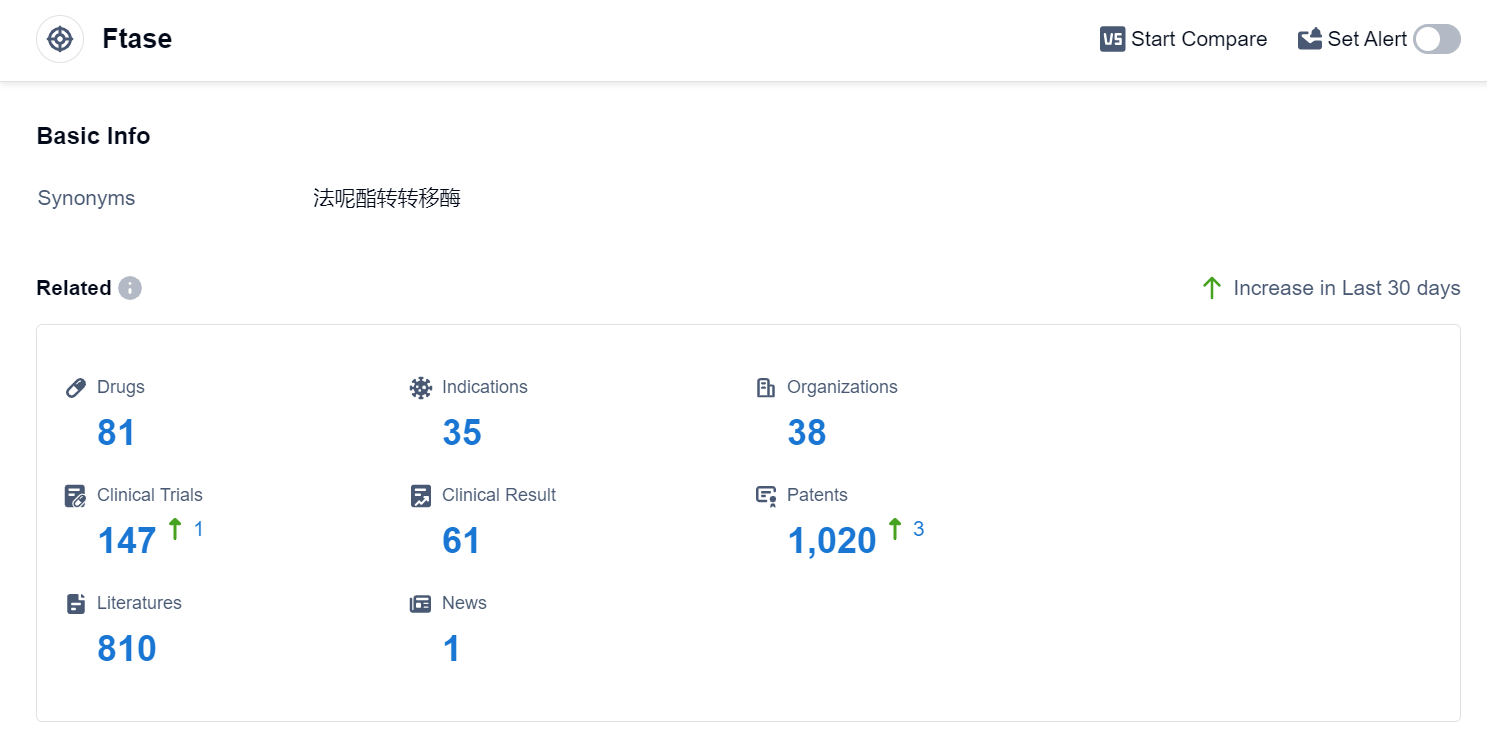
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 81 Ftase drugs worldwide, from 38 organizations, covering 35 indications, and conducting 147 clinical trials.
The analysis of the current competitive landscape of target Ftase reveals that companies like Eiger BioPharmaceuticals, Inc., Merck & Co., Inc., Kura Oncology, Inc., and Johnson & Johnson are leading the R&D progress. Drugs targeting Ftase have been approved for indications such as Breast Cancer, Lung Cancer, Progeria, and Liver Cancer. Small molecule drugs and Synthetic peptides are the drug types progressing most rapidly. The European Union and the United States are the countries/locations with the highest development phases. The future development of target Ftase is promising, with ongoing research and development efforts focused on advancing the therapeutic potential of drugs targeting Ftase.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Lonafarnib is a small molecule drug developed by Merck Sharp & Dohme Corp. It targets Ftase and has received approval for the treatment of Progeria and Hepatitis D. Its approval in the United States, along with its breakthrough therapy and orphan drug designations, highlights its potential in addressing unmet medical needs in rare diseases and infectious conditions. Lonafarnib's first approval date in November 2020 marks a significant milestone in the field of biomedicine.