Fosaprepitant Dimeglumine Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Fosaprepitant Dimeglumine's R&D Progress
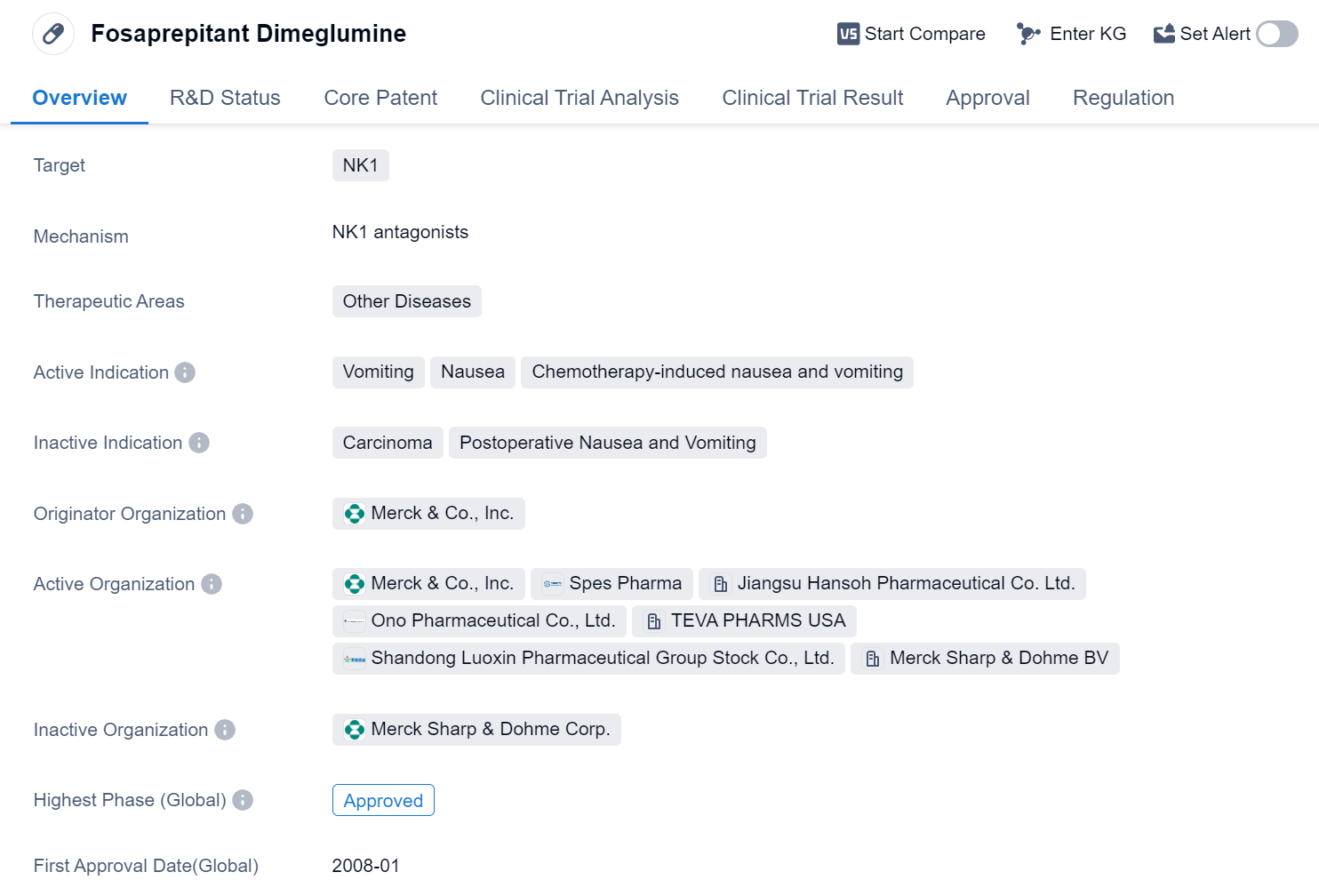
Fosaprepitant Dimeglumine is a small molecule drug that falls under the therapeutic area of Other Diseases in the field of biomedicine. It specifically targets the NK1 receptor and is primarily used to treat vomiting and nausea, particularly those induced by chemotherapy.
The drug was developed by Merck & Co., Inc., a renowned pharmaceutical organization. Fosaprepitant Dimeglumine has successfully completed the highest phase of clinical trials and has been approved for use globally. Its first approval was granted in January 2008 by the European Union.
The approval process for Fosaprepitant Dimeglumine was expedited through priority review, indicating its potential significance in addressing the medical needs of patients suffering from chemotherapy-induced nausea and vomiting. This regulatory designation suggests that the drug demonstrated promising results in clinical trials and may offer substantial benefits compared to existing treatment options.
Chemotherapy-induced nausea and vomiting are common side effects experienced by cancer patients undergoing chemotherapy. These symptoms can significantly impact a patient's quality of life and may even lead to treatment discontinuation. Fosaprepitant Dimeglumine, by targeting the NK1 receptor, aims to alleviate these distressing symptoms and improve patient outcomes.
As a small molecule drug, Fosaprepitant Dimeglumine is likely to have a well-defined chemical structure, allowing for efficient manufacturing and formulation processes. This characteristic may contribute to its widespread availability and accessibility for patients in need.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Fosaprepitant Dimeglumine: NK1 antagonists
NK1 antagonists are a type of drug that act as antagonists to the neurokinin-1 (NK1) receptor. The NK1 receptor is a protein found in the central nervous system and is involved in transmitting signals related to pain, inflammation, and stress. By blocking the NK1 receptor, NK1 antagonists can inhibit the binding of substance P, a neurotransmitter that plays a role in pain perception and inflammation. This inhibition can help alleviate symptoms associated with various conditions, including chemotherapy-induced nausea and vomiting, postoperative nausea and vomiting, and certain psychiatric disorders. NK1 antagonists are commonly used in combination with other antiemetic drugs to prevent and manage nausea and vomiting caused by chemotherapy.
Drug Target R&D Trends for Fosaprepitant Dimeglumine
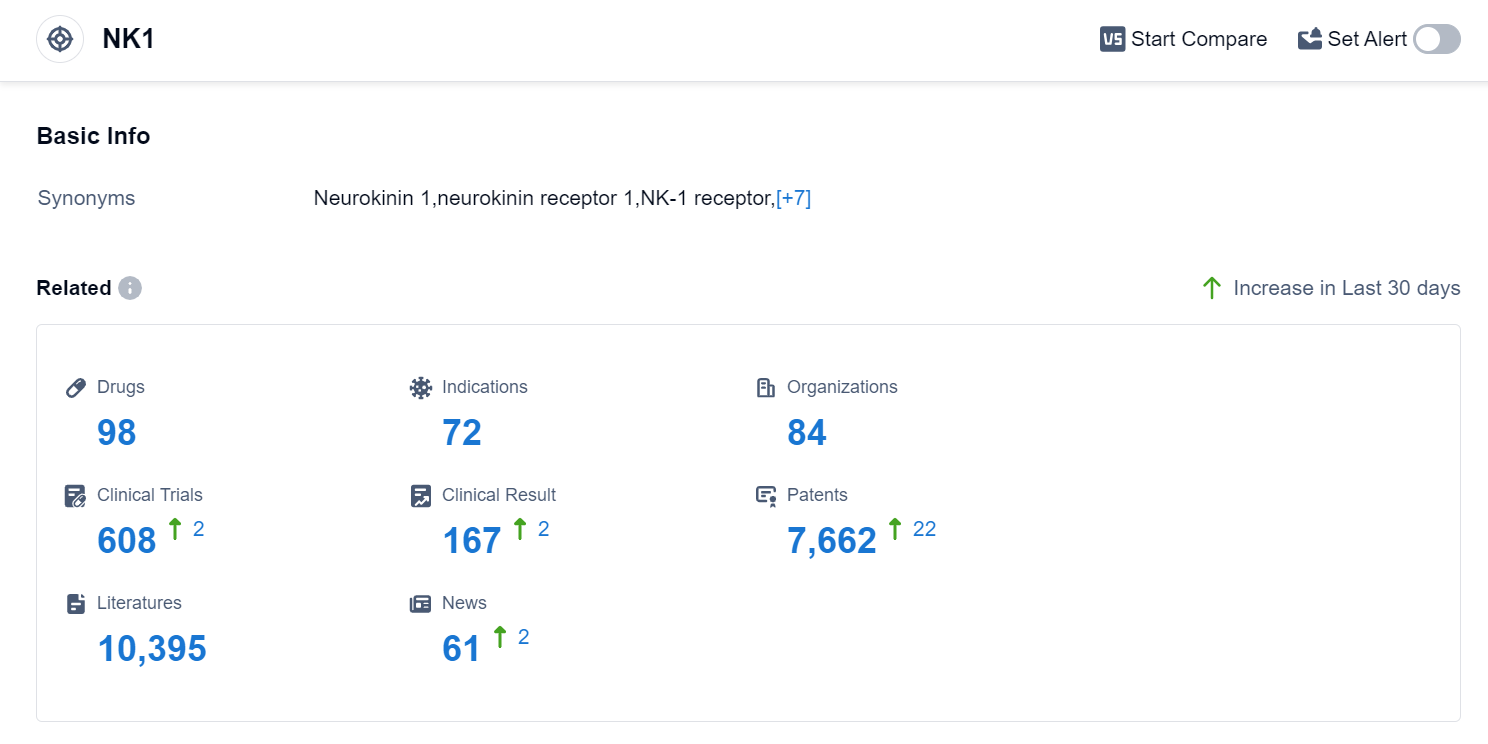
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 98 NK1 drugs worldwide, from 84 organizations, covering 72 indications, and conducting 608 clinical trials.
Based on the analysis of the data, the current competitive landscape of target NK1 in the pharmaceutical industry is characterized by the presence of multiple companies with varying stages of development and drug types. Merck & Co., Inc. is leading in terms of the highest stage of development, with multiple approved drugs. The indications with the highest number of approved drugs are chemotherapy-induced nausea and vomiting, nausea, and vomiting. Small molecule drugs are progressing rapidly, indicating intense competition in the market. The United States, European Union, and Japan are the leading countries/locations in the development of drugs under target NK1, with China also showing progress.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Fosaprepitant Dimeglumine is an approved small molecule drug developed by Merck & Co., Inc. It targets the NK1 receptor and is primarily used to treat vomiting and nausea induced by chemotherapy. Its approval in the global market, along with its priority review designation, highlights its potential as an effective therapeutic option for patients suffering from chemotherapy-induced nausea and vomiting.