An In-depth Analysis of Metyrapone's R&D Progress and Mechanism of Action
Metyrapone's R&D Progress
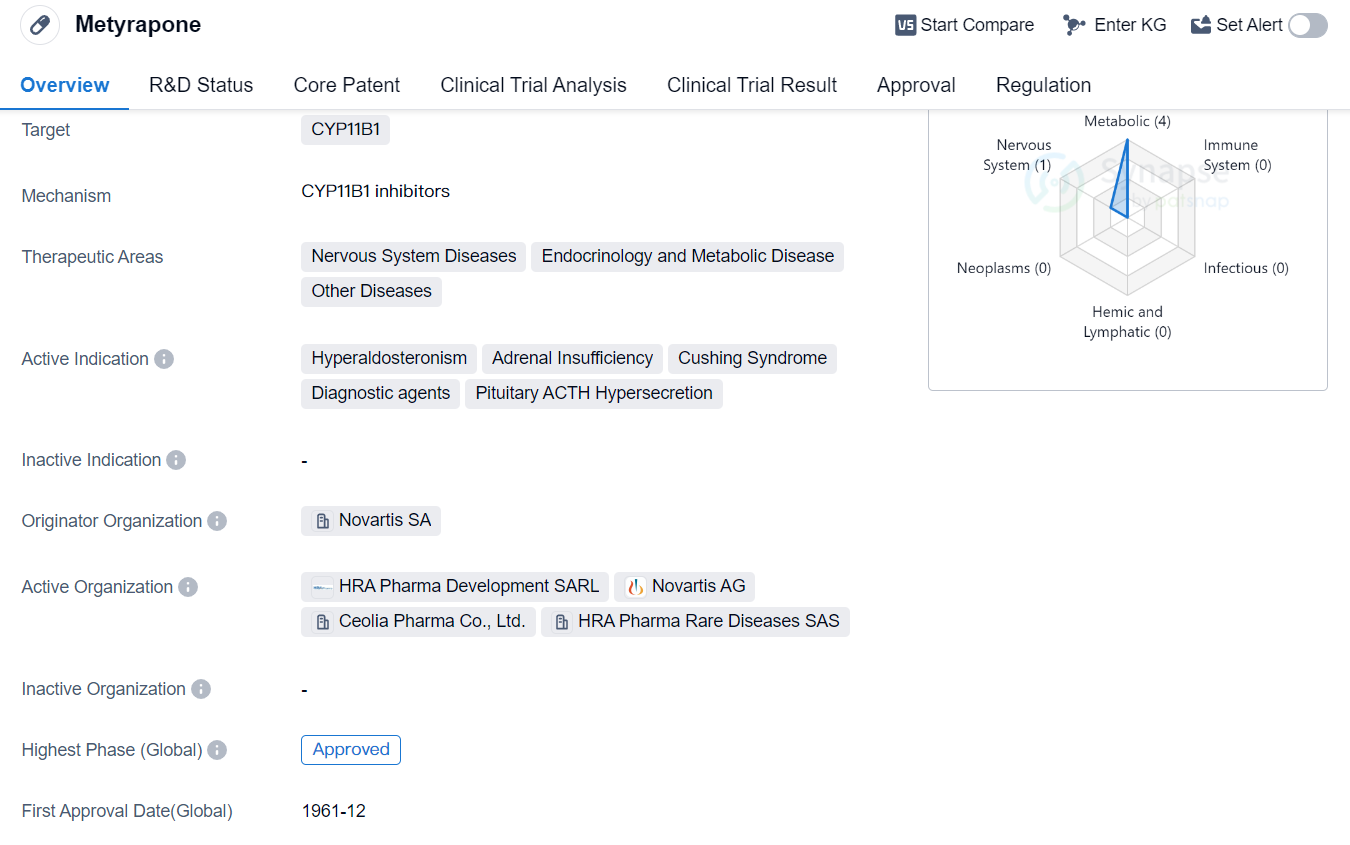
Metyrapone is a small molecule drug that targets the enzyme CYP11B1. It is primarily used in the treatment of various diseases related to the nervous system, endocrinology, and metabolic disorders. Additionally, it has been approved for use in other diseases as well. The active indications for Metyrapone include hyperaldosteronism, adrenal insufficiency, Cushing syndrome, diagnostic agents, and pituitary ACTH hypersecretion.
The drug was first approved for use in the United States in December 1961. It is important to note that Metyrapone is classified as an orphan drug, indicating that it is intended to treat rare diseases or conditions. This classification provides certain regulatory benefits and incentives to the drug's manufacturer, Novartis SA.
As a small molecule drug, Metyrapone is designed to interact with the CYP11B1 enzyme. This enzyme plays a crucial role in the synthesis of cortisol, a hormone involved in various physiological processes. By targeting CYP11B1, Metyrapone can modulate cortisol production, making it an effective treatment option for conditions such as hyperaldosteronism, adrenal insufficiency, and Cushing syndrome.
Hyperaldosteronism is a condition characterized by excessive production of aldosterone, a hormone that regulates electrolyte balance. Metyrapone can help reduce aldosterone levels, thereby managing the symptoms associated with this condition. Adrenal insufficiency, on the other hand, refers to the inadequate production of cortisol by the adrenal glands. Metyrapone can stimulate cortisol production, helping to restore normal hormonal balance.
Cushing syndrome is a disorder caused by prolonged exposure to high levels of cortisol. Metyrapone can inhibit cortisol synthesis, effectively managing the symptoms of this condition. Additionally, Metyrapone is used as a diagnostic agent to assess the functioning of the hypothalamic-pituitary-adrenal (HPA) axis, which is involved in cortisol regulation.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Metyrapone: CYP11B1 inhibitor
CYP11B1 inhibitors are a type of medication that specifically target and inhibit the activity of the enzyme CYP11B1. CYP11B1 is an enzyme that plays a crucial role in the synthesis of cortisol, a hormone involved in regulating various physiological processes in the body. By inhibiting CYP11B1, these inhibitors can reduce the production of cortisol.
From a biomedical perspective, CYP11B1 inhibitors are used in the treatment of conditions such as Cushing's syndrome, which is characterized by excessive cortisol production. By blocking the activity of CYP11B1, these inhibitors can help normalize cortisol levels and alleviate the symptoms associated with this condition.
It is important to note that CYP11B1 inhibitors should be used under medical supervision, as they can have potential side effects and interactions with other medications. Additionally, the use of these inhibitors may require regular monitoring of cortisol levels to ensure optimal therapeutic outcomes.
Drug Target R&D Trends for Metyrapone
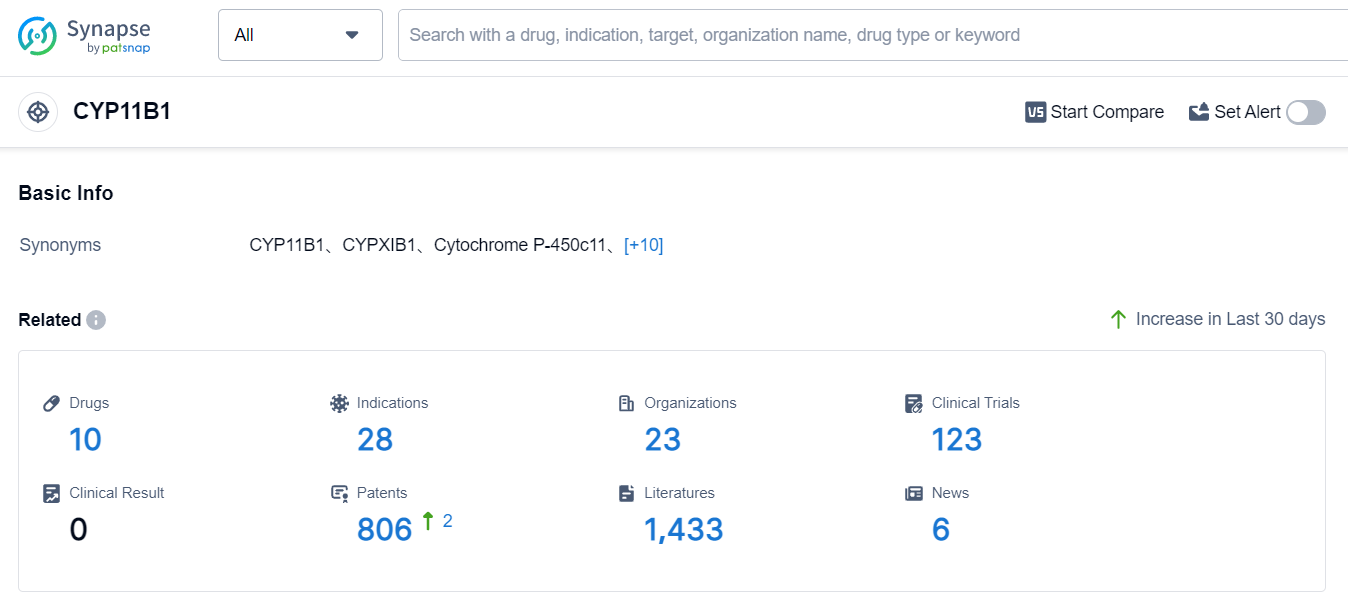
According to Patsnap Synapse, as of 3 Sep 2023, there are a total of 10 CYP11B1 drugs worldwide, from 23 organizations, covering 28 indications, and conducting 123 clinical trials.
The analysis of the target CYP11B1 reveals a competitive landscape with multiple companies actively developing drugs. Novartis AG has shown the highest stage of development and significant R&D progress. The indications for drugs targeting CYP11B1 cover a wide range of medical conditions, indicating the potential for therapeutic applications. Small molecule drugs and diagnostic radiopharmaceuticals are the drug types progressing most rapidly, suggesting intense competition. The countries/locations that are developing fastest include the United States, Japan, European Union, and China. Overall, the target CYP11B1 presents opportunities for innovation and competition in the pharmaceutical industry.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Metyrapone has been approved for use in various countries and has demonstrated efficacy in treating several diseases related to the nervous system, endocrinology, and metabolic disorders. Its classification as an orphan drug highlights its importance in addressing rare conditions. As the originator organization, Novartis SA has played a significant role in the development and commercialization of this drug.