Magrolimab: Detailed Review of its Transformative R&D Success
Magrolimab's R&D Progress
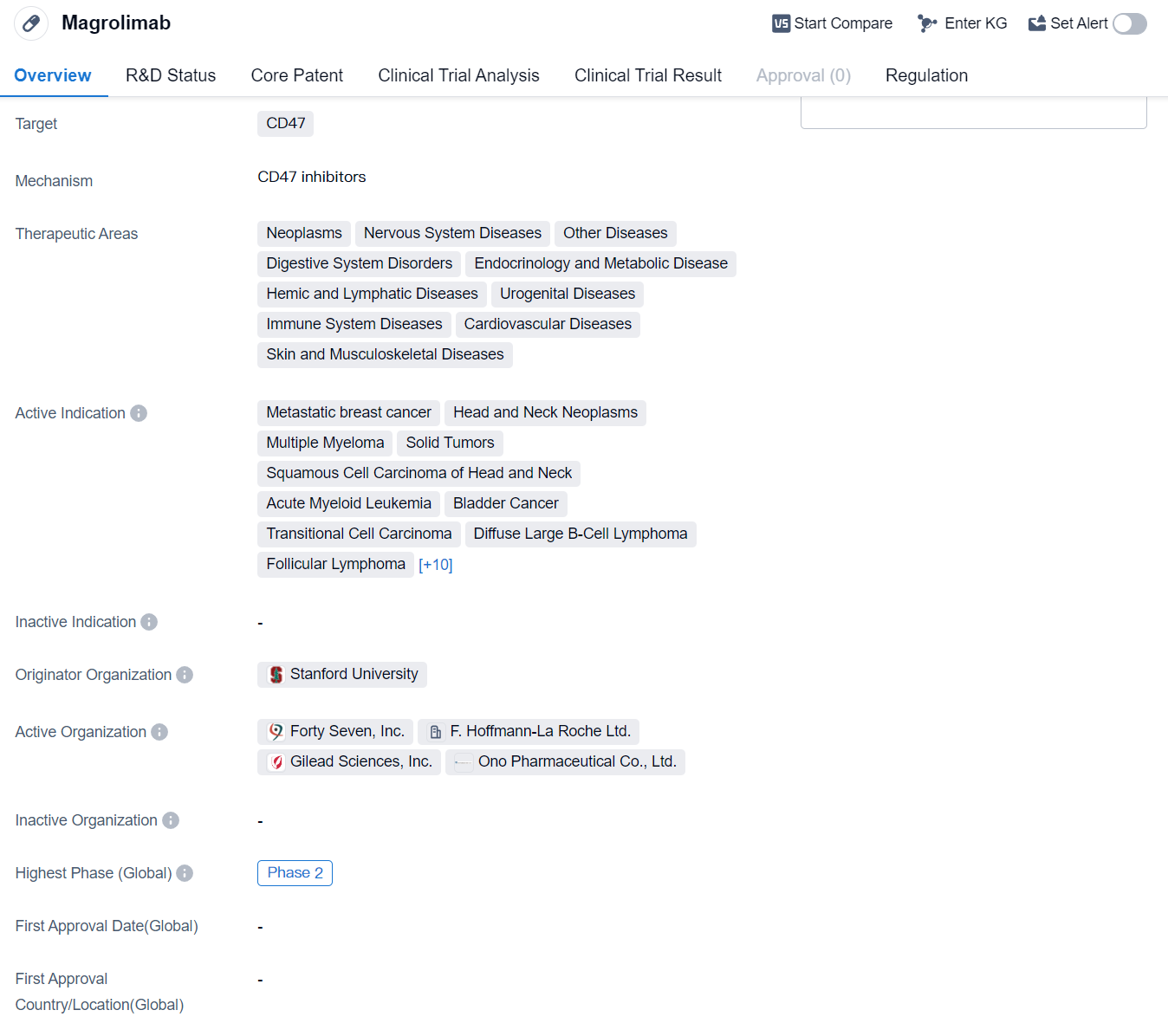
Magrolimab is a monoclonal antibody drug that targets CD47, a protein that is overexpressed in various diseases. It has shown potential therapeutic benefits in a wide range of therapeutic areas, including neoplasms, nervous system diseases, digestive system disorders, endocrinology and metabolic disease, hemic and lymphatic diseases, urogenital diseases, immune system diseases, cardiovascular diseases, and skin and musculoskeletal diseases.
The drug is currently being investigated in multiple indications, including metastatic breast cancer, head and neck neoplasms, multiple myeloma, solid tumors, squamous cell carcinoma of head and neck, acute myeloid leukemia, bladder cancer, transitional cell carcinoma, diffuse large B-cell lymphoma, follicular lymphoma, non-Hodgkin lymphoma, colorectal cancer, pancreatic cancer, myelodysplastic syndromes, ovarian cancer, hematologic neoplasms, brain metastases, ependymoma, medulloblastoma, and other neoplasms.
Magrolimab was originated by Stanford University and has reached the highest phase of development, which is Phase 2. This indicates that the drug has already undergone initial testing for safety and efficacy in a relatively small number of patients. The fact that it has reached Phase 2 suggests that it has shown promising results in earlier stages of development.
Furthermore, Magrolimab has been granted orphan drug status, which is a regulatory designation given to drugs that are intended to treat rare diseases or conditions. This status provides certain incentives and benefits to the drug developer, such as market exclusivity and financial incentives, to encourage the development of treatments for rare diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Magrolimab: CD47 inhibitor
CD47 inhibitors are a type of medication that target and block the CD47 protein. CD47 is a cell surface protein that is involved in various cellular processes, including immune regulation and cell signaling. In the context of biomedicine, CD47 inhibitors are being studied for their potential therapeutic applications in cancer treatment.
CD47 is often overexpressed on the surface of cancer cells, allowing them to evade the immune system's detection and destruction. By inhibiting CD47, these inhibitors can enhance the immune response against cancer cells, promoting their elimination. This approach is known as immunotherapy and holds promise for improving the effectiveness of cancer treatments.
CD47 inhibitors work by binding to CD47 and preventing its interaction with another protein called SIRPα. This interaction between CD47 and SIRPα sends a "don't eat me" signal to immune cells, inhibiting their ability to engulf and destroy cancer cells. By blocking this signal, CD47 inhibitors help to unleash the immune system's ability to recognize and eliminate cancer cells.
Research on CD47 inhibitors is still ongoing, and several drugs in this class are currently being developed and tested in clinical trials. These inhibitors have shown promising results in preclinical studies, demonstrating their potential to enhance the anti-cancer immune response and improve patient outcomes. However, further research is needed to fully understand their safety, efficacy, and optimal use in cancer treatment.
Drug Target R&D Trends for Magrolimab
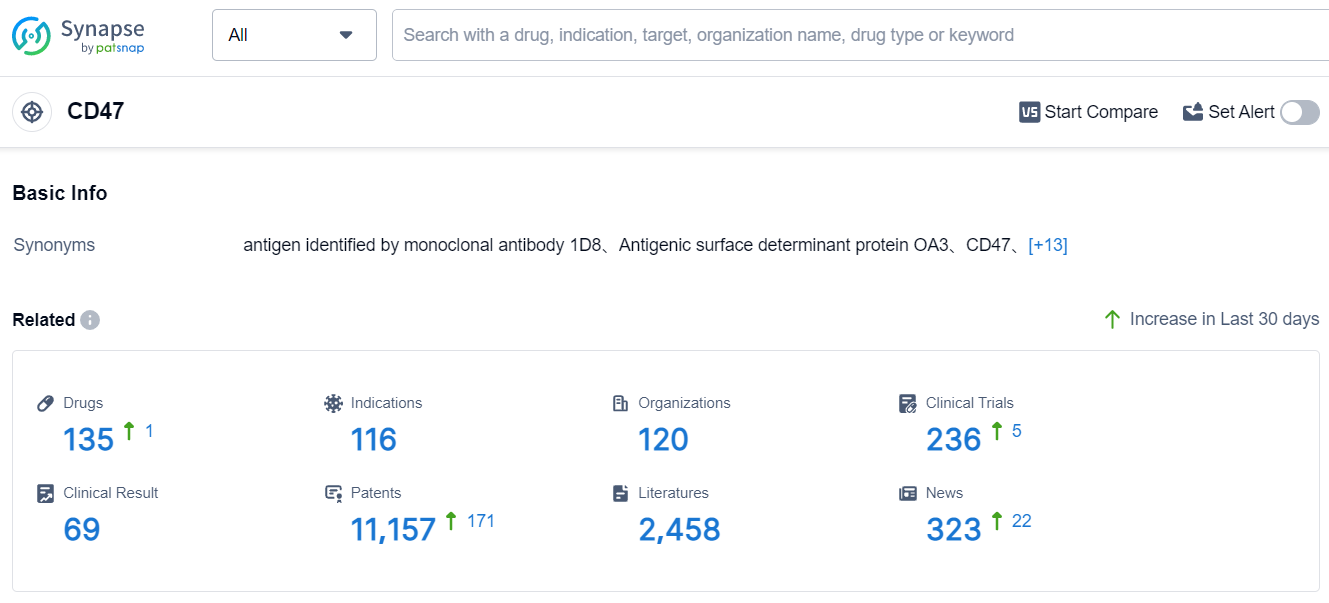
According to Patsnap Synapse, as of 4 Sep 2023, there are a total of 135 CD47 drugs worldwide, from 120 organizations, covering 116 indications, and conducting 236 clinical trials.
The analysis of target CD47 reveals that Innovent Biologics, Inc., I-MAB Biopharma Co., Ltd., EpicentRx, Inc., and SciClone Pharmaceuticals (Holdings) Ltd. are the companies growing fastest under this target. The highest stage of development for target CD47 is Phase 3, indicating advanced R&D progress. Drugs under target CD47 have been approved for indications such as Myelodysplastic Syndromes, Small Cell Lung Cancer, Neuroendocrine Tumors, and various other cancers. Monoclonal antibody, Bispecific antibody, Fusion protein, and Small molecule drug are the drug types progressing most rapidly. China and the United States are the countries developing fastest under this target, with China showing significant progress in R&D. The competitive landscape for target CD47 is intense, with multiple companies and drug types vying for development and approval. The future development of target CD47 holds promise for the treatment of various cancers and other indications.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Magrolimab is a monoclonal antibody drug that targets CD47 and has shown potential therapeutic benefits in a wide range of diseases. It is currently being investigated in multiple indications, including various types of cancer and other neoplasms. The drug has been originated by Stanford University and has reached Phase 2 of development. Additionally, it has been granted orphan drug status, indicating its potential to address unmet medical needs in rare diseases.