An Overview of Ipsen’s Drug Pipeline | R&D Progress
Ipsen SA is a pharmaceutical organization that was founded in 1929 and is based in France. The company operates in the field of biomedicine and has a diverse portfolio of drugs targeting various therapeutic areas. Ipsen Group focused on drug innovation and oncology, neuroscience and rare diseases, with a strong track record in the multi-health business. Committed to becoming the world's leading biopharmaceutical company in the field of innovation and specialty treatment. In this report, we will analyze the distribution of therapeutic areas, the most frequently developed targets, and the pipeline of Ipsen SA.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Ipsen SA.
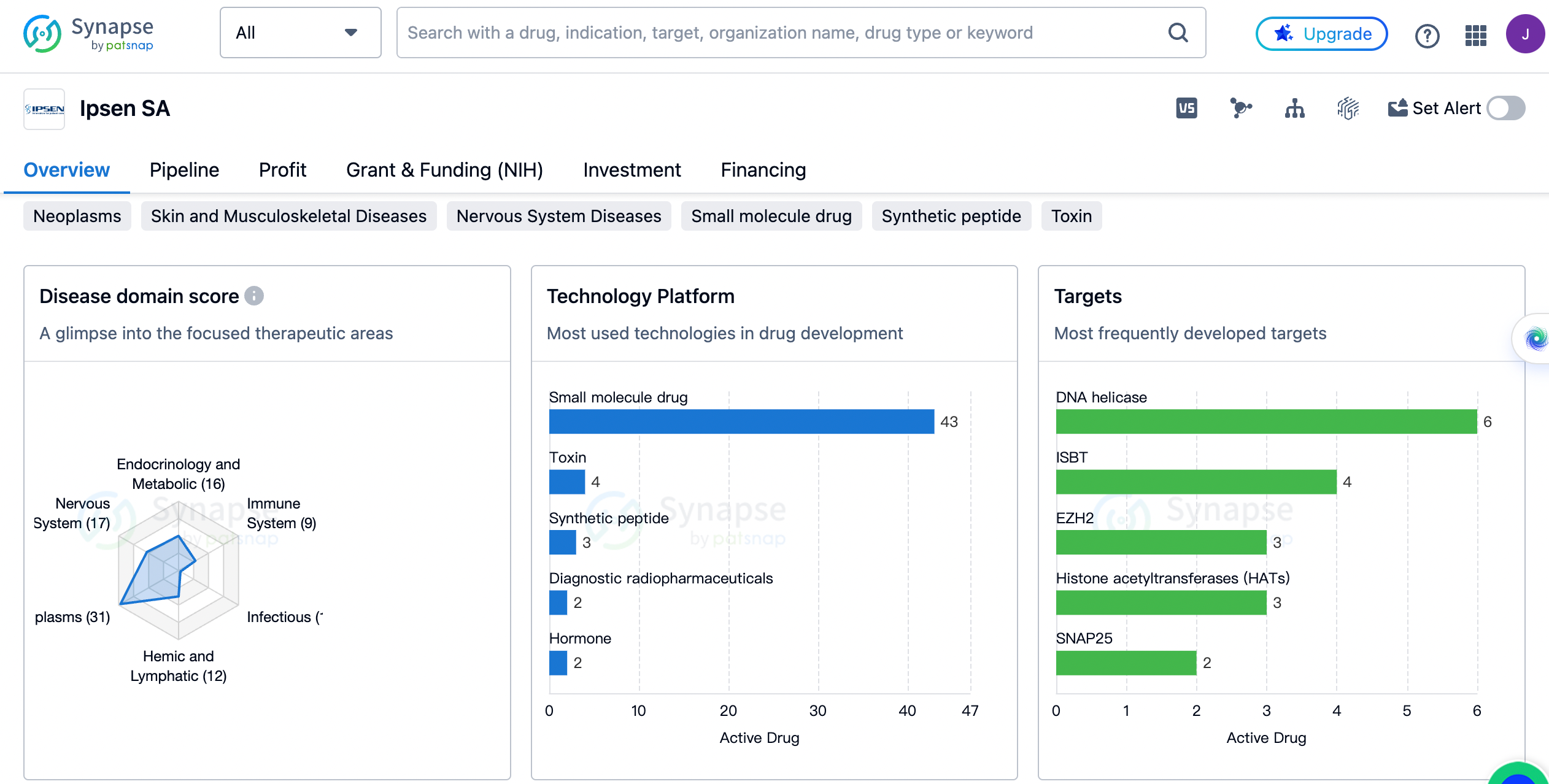
An overview of the distribution of therapeutic areas
The organization has a strong focus on neoplasms, with 31 drugs developed in this area. Nervous system diseases and endocrinology and metabolic diseases are also key areas of interest for Ipsen SA, with 17 and 16 drugs respectively. Other therapeutic areas that Ipsen SA has developed drugs for include skin and musculoskeletal diseases, digestive system disorders, hemic and lymphatic diseases, immune system diseases, urogenital diseases, congenital disorders, cardiovascular diseases, respiratory diseases, eye diseases, otorhinolaryngologic diseases, and infectious diseases.
The most frequently developed targets by Ipsen
DNA helicase is the most targeted protein, with 6 drugs developed to interact with it. Other frequently targeted proteins include ISBT, EZH2, and histone acetyltransferases (HATs), each with 3 drugs developed. Ipsen SA has also developed drugs targeting proteins such as SNAP25, MC4R, DOT1L, SSTR2, NTCP, ALK2, AChR, COX-1 + COX-2, GHSR, GHR, D2 receptor + SSTR2, RARγ, GnRHR, IGF-1R, NOS2, and TOP1.
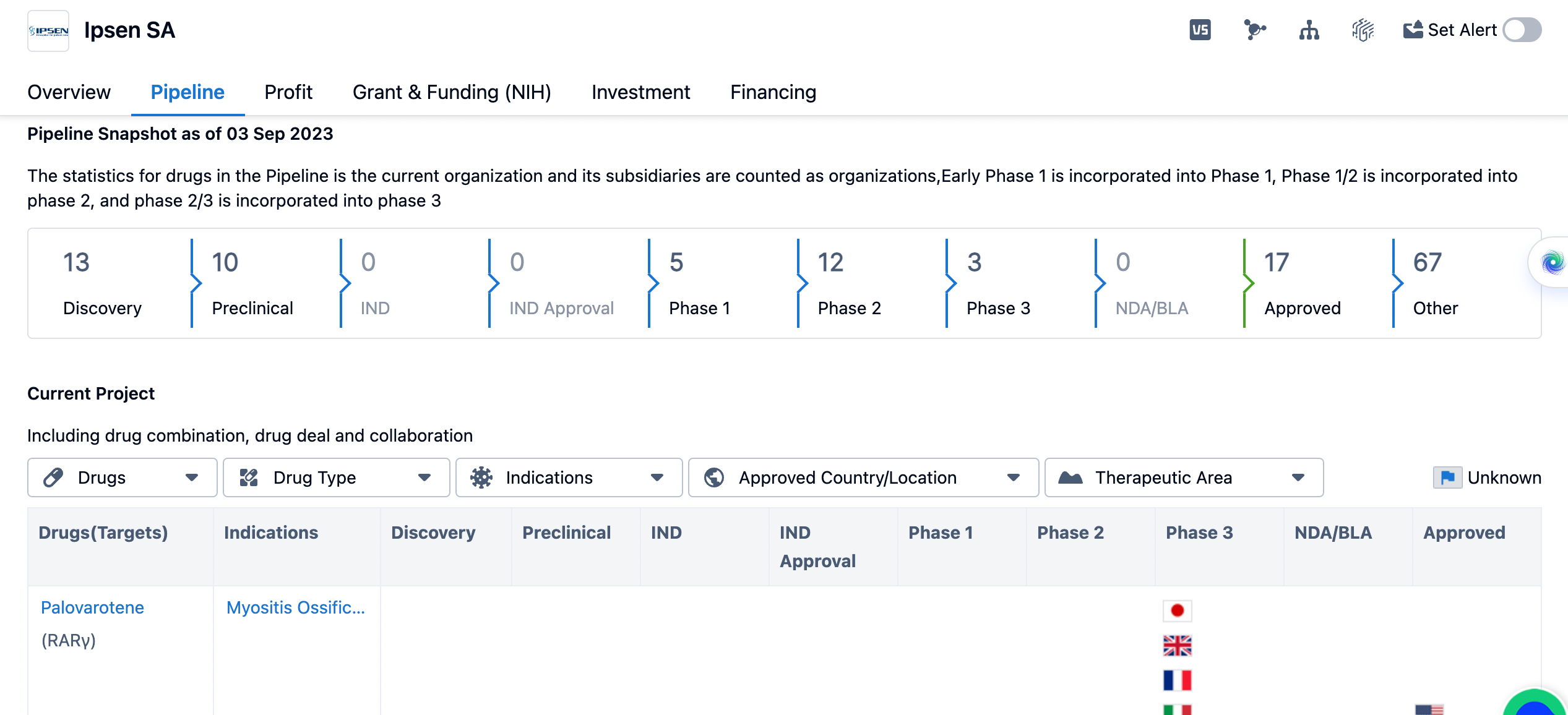
An overview of Ipsen's pipeline
The organization has 13 drugs in the discovery phase, indicating ongoing research and development efforts. Additionally, Ipsen SA has 10 drugs in the preclinical phase, which suggests that these drugs are undergoing testing in laboratory settings. In terms of clinical development, Ipsen SA has 5 drugs in phase 1, 12 drugs in phase 2, and 3 drugs in phase 3. Phase 1 trials involve testing the safety and dosage of a drug in a small group of healthy volunteers or patients. Phase 2 trials assess the effectiveness and side effects of a drug in a larger group of patients. Phase 3 trials involve a larger number of patients and aim to confirm the effectiveness and monitor side effects in a real-world setting.
There are no drugs from Ipsen SA in the NDA/BLA (New Drug Application/Biologics License Application) phase, which is the stage where a company submits a comprehensive application to regulatory authorities for approval to market a drug. Ipsen SA has 17 drugs that have been approved, indicating successful regulatory clearance for these products. Additionally, there are 67 drugs listed under the "Other" category, which could include drugs in various stages of development or those that have been discontinued.
In summary, Ipsen SA is a pharmaceutical organization with a long history in the industry. The company has a diverse portfolio of drugs targeting various therapeutic areas, with a strong focus on neoplasms, nervous system diseases, and endocrinology and metabolic diseases. Ipsen SA has developed drugs targeting a range of proteins, with DNA helicase being the most frequently targeted. The organization has an active pipeline, with drugs in the discovery, preclinical, phase 1, phase 2, and phase 3 stages. Ipsen SA has successfully obtained approval for 17 drugs, indicating its commitment to bringing innovative treatments to market.