An In-depth Analysis of Milrinone Lactate's R&D Progress and Mechanism of Action on Drug Target
Milrinone Lactate's R&D Progress
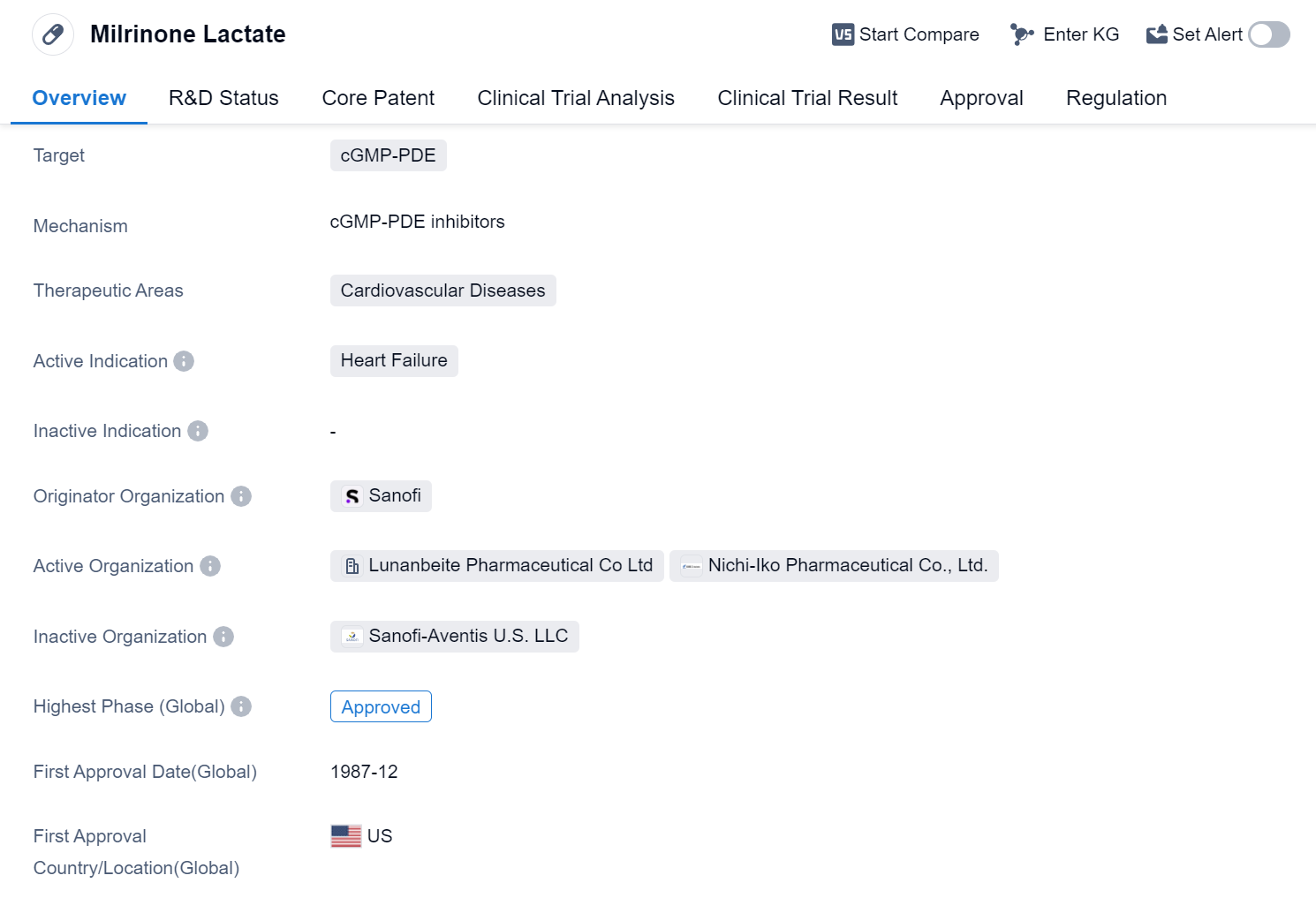
Milrinone Lactate is a small molecule drug that falls under the therapeutic area of cardiovascular diseases. It specifically targets cGMP-PDE, which is an enzyme involved in the regulation of cyclic guanosine monophosphate (cGMP) levels. This drug is primarily indicated for the treatment of heart failure.
The originator organization of Milrinone Lactate is Sanofi, a well-known pharmaceutical company. It is important to note that this drug has reached the highest phase of approval globally. The first approval for Milrinone Lactate was granted in December 1987 in the United States.
In terms of regulatory status, Milrinone Lactate has undergone priority review, indicating that it was considered a high-priority drug due to its potential benefits in treating heart failure. Additionally, it has been designated as an orphan drug, which suggests that it is intended to treat a rare disease or condition.
Milrinone Lactate has been widely used in the treatment of heart failure, a condition characterized by the heart's inability to pump blood effectively. By targeting cGMP-PDE, this drug helps to increase the levels of cGMP, which in turn leads to vasodilation and improved cardiac contractility. These effects can help alleviate the symptoms of heart failure and improve patients' quality of life.
Since its approval in 1987, Milrinone Lactate has been an important therapeutic option for patients suffering from heart failure. The approval of Milrinone Lactate as an orphan drug highlights the need for effective treatments for heart failure, particularly in cases where the condition is rare.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Milrinone Lactate: cGMP-PDE inhibitors
cGMP-PDE inhibitors are a type of drug that act by inhibiting the enzyme phosphodiesterase (PDE) that specifically targets cyclic guanosine monophosphate (cGMP). From a biomedical perspective, cyclic guanosine monophosphate (cGMP) is a signaling molecule involved in various physiological processes, including smooth muscle relaxation, platelet aggregation, and regulation of blood vessel dilation. Phosphodiesterase (PDE) enzymes degrade cGMP, leading to a decrease in its concentration and subsequent physiological effects.
cGMP-PDE inhibitors, such as sildenafil (Viagra) and tadalafil (Cialis), work by blocking the action of PDE enzymes, thereby preventing the breakdown of cGMP. By maintaining elevated levels of cGMP, these inhibitors promote smooth muscle relaxation, particularly in the blood vessels of the penis, leading to increased blood flow and facilitating erectile function. These inhibitors are commonly used to treat erectile dysfunction (impotence) in men.
Additionally, cGMP-PDE inhibitors have other therapeutic applications. For example, they can be used to treat pulmonary arterial hypertension (PAH), a condition characterized by high blood pressure in the arteries of the lungs. By dilating the pulmonary blood vessels, cGMP-PDE inhibitors help reduce the workload on the heart and improve exercise capacity in individuals with PAH.
It's important to note that cGMP-PDE inhibitors should be used under medical supervision, as they can have potential side effects and interactions with other medications.
Drug Target R&D Trends for Milrinone Lactate
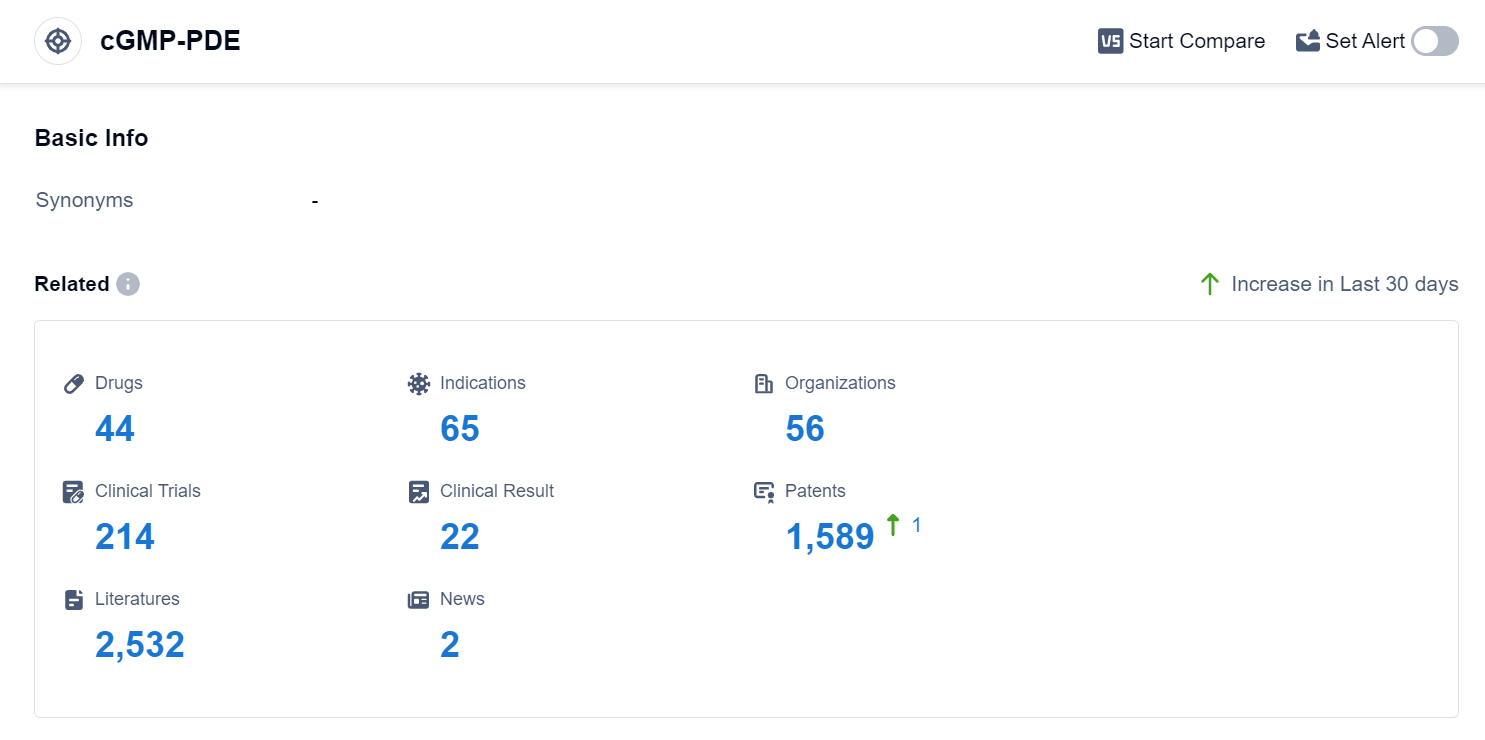
According to Patsnap Synapse, as of 4 Sep 2023, there are a total of 44 cGMP-PDE drugs worldwide, from 56 organizations, covering 65 indications, and conducting 214 clinical trials.
The analysis of the current competitive landscape of target cGMP-PDE reveals that multiple companies are growing rapidly, with a focus on small molecule drugs. The highest stage of development is the approved phase, with a significant number of drugs targeting respiratory and cardiovascular diseases. Japan, China, and the United States are leading in terms of drug development, with China showing notable progress. The future development of target cGMP-PDE is expected to continue focusing on small molecule drugs, with potential competition from biosimilars. Further analysis of individual companies, indications, drug types, and countries/locations would provide a more detailed understanding of the market dynamics and future opportunities in this target area.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Milrinone Lactate is a small molecule drug developed by Sanofi for the treatment of heart failure. It targets cGMP-PDE and has received approval in both the United States and China. Its regulatory status includes priority review and orphan drug designation. Since its approval in 1987, Milrinone Lactate has played a significant role in managing heart failure and improving patients' outcomes.