An In-depth Analysis of Thioguanine's R&D Progress and Mechanism of Action on Drug Target
Thioguanine's R&D Progress
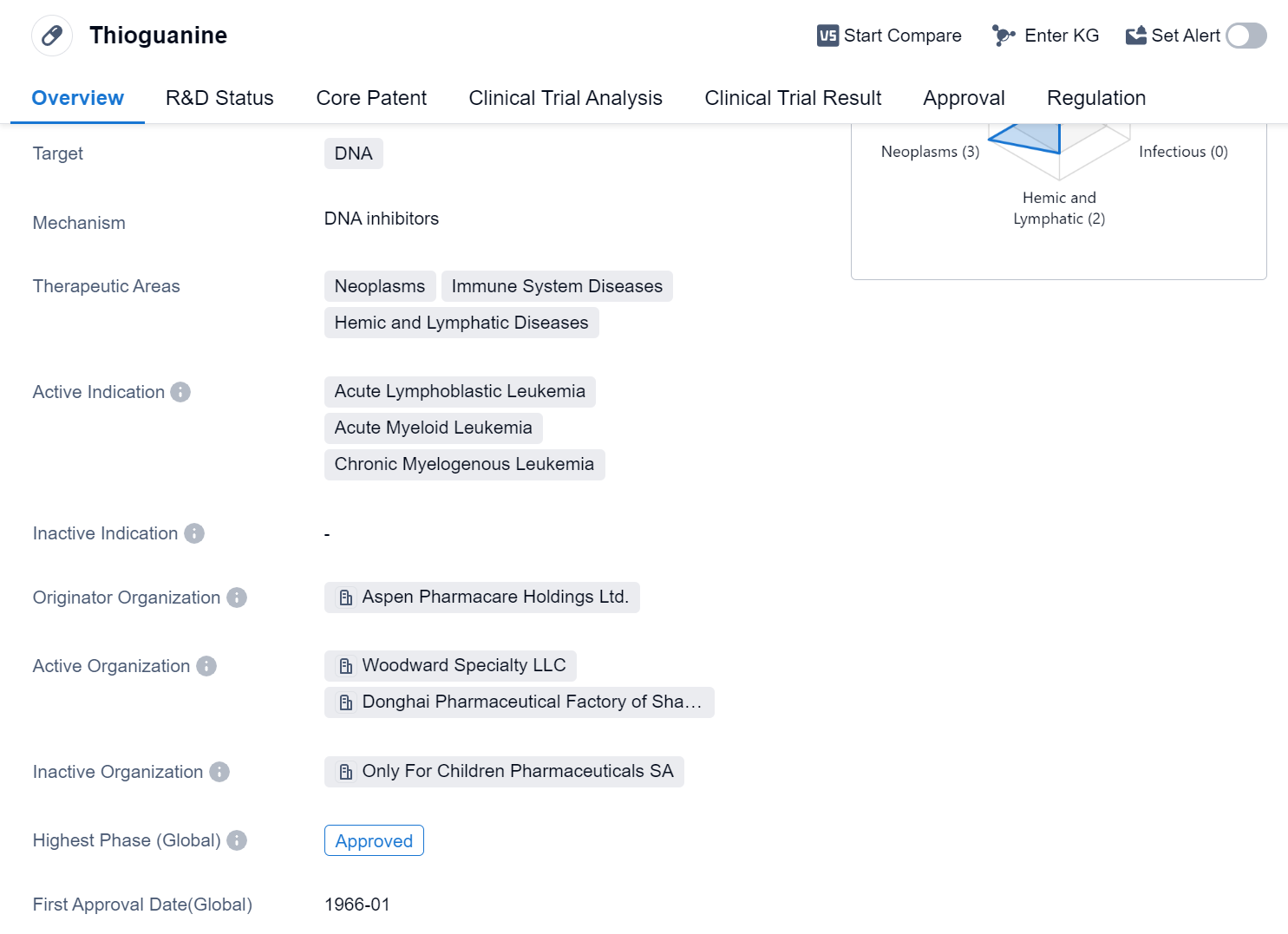
Thioguanine is a small molecule drug that targets DNA and is used in the treatment of various neoplasms, immune system diseases, and hemic and lymphatic diseases. It has been approved for the treatment of acute lymphoblastic leukemia, acute myeloid leukemia, and chronic myelogenous leukemia. The drug was first approved in the United States in January 1966 and is regulated as an orphan drug.
Thioguanine is developed by Aspen Pharmacare Holdings Ltd., a pharmaceutical company specializing in the production of generic and branded medicines. As a small molecule drug, it acts by interfering with the synthesis of DNA, which is crucial for the growth and division of cancer cells. By targeting DNA, thioguanine disrupts the replication process and inhibits the proliferation of cancer cells.
The drug has reached the highest phase of approval globally. Its approval as an orphan drug suggests that it is intended for the treatment of rare diseases or conditions that affect a small number of patients. This designation often provides incentives for pharmaceutical companies to develop drugs for such conditions.
Thioguanine's therapeutic areas include neoplasms, which refer to abnormal growths of cells that can be cancerous, immune system diseases, which involve dysregulation of the immune response, and hemic and lymphatic diseases, which affect the blood and lymphatic systems. These broad therapeutic areas highlight the potential of thioguanine in treating a range of conditions related to these disease categories.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Thioguanine: DNA inhibitors
DNA inhibitors are substances that interfere with the replication or transcription of DNA, thereby preventing the normal functioning of the DNA molecule. These inhibitors can be used in various fields, including biomedicine and clinical research.
From a biomedical perspective, DNA inhibitors are often used in cancer treatment. Cancer cells have uncontrolled growth and division, and DNA inhibitors can target the DNA replication process in these cells, inhibiting their ability to divide and proliferate. By blocking DNA replication, these inhibitors can help slow down or halt the growth of cancer cells, ultimately leading to their death.
There are different types of DNA inhibitors, including chemotherapeutic drugs that directly bind to DNA and disrupt its structure, preventing proper replication or transcription. Examples of DNA inhibitors used in cancer treatment include cisplatin and doxorubicin.
In addition to cancer treatment, DNA inhibitors can also be used in research to study DNA replication, transcription, and other cellular processes. By selectively inhibiting DNA, scientists can gain insights into the mechanisms and functions of DNA, as well as develop new therapeutic strategies.
Overall, DNA inhibitors play a crucial role in both biomedical research and clinical applications, particularly in the field of cancer treatment. They offer a targeted approach to disrupt DNA replication and transcription, providing potential avenues for therapeutic intervention.
Drug Target R&D Trends for Thioguanine
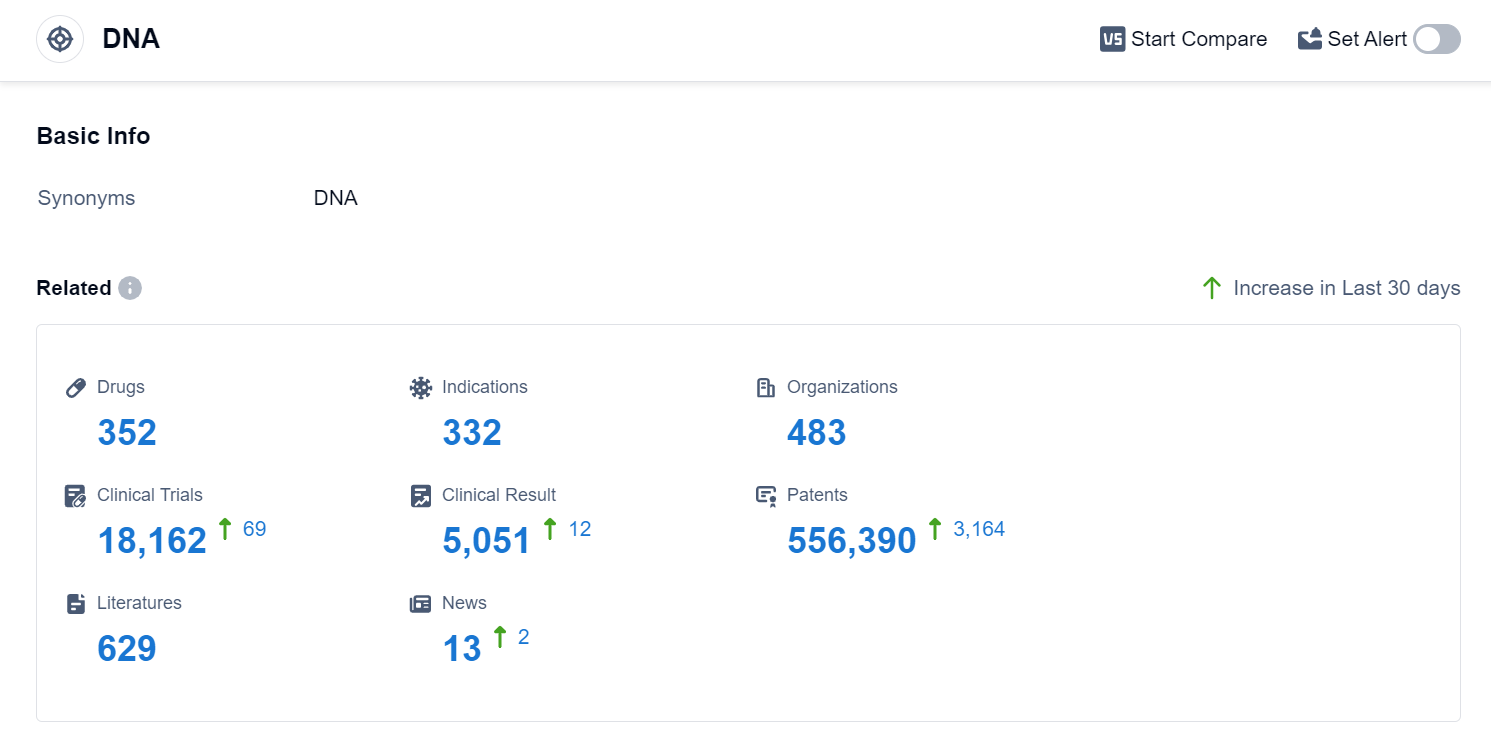
According to Patsnap Synapse, as of 4 Sep 2023, there are a total of 352 DNA drugs worldwide, from 483 organizations, covering 332 indications, and conducting 18162 clinical trials.
The analysis of the current competitive landscape and future development of target DNA in the pharmaceutical industry reveals that Pfizer Inc., Novartis AG, Baxter International, Inc., Takeda Pharmaceutical Co., Ltd., and Johnson & Johnson are the companies growing fastest under the current target. The most common indications for drugs targeting the DNA include lymphoma, ovarian cancer, breast cancer, neoplasms, and Hodgkin's lymphoma. Small molecule drugs, monoclonal antibodies, and ADCs are progressing most rapidly under the current targets. China, the United States, Japan, and the European Union are the countries/locations developing fastest under the current targets, with China showing significant progress. Overall, the target DNA presents a promising area for the development of innovative drugs in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, thioguanine is an approved small molecule drug that targets DNA and is used in the treatment of various cancers and immune system diseases. Its approval as an orphan drug and its efficacy in treating different types of leukemia make it a valuable option for patients with these conditions. As an expert in the pharmaceutical industry, it is important to consider the potential benefits and limitations of thioguanine in the context of other available treatment options and patient needs.