Analysis of Amgen’s Drug Development Pipeline | R&D Progress | Drug Target
Amgen Inc. is a biotechnology company dedicated to providing biological therapeutics to patients with serious diseases by discovering, developing, manufacturing, and delivering innovative human therapies. Amgen focuses on areas of imperfect medical needs and uses its expertise to strive for solutions that improve health and measurably improve people's lives. As a pioneer in biotechnology, Amgen has grown to become one of the world's leading independent biotechnology companies, covering millions of patients worldwide and developing a pipeline of drugs with breakthrough potential. Amgen was incorporated in California in 1980 and became a Delaware corporation in 1987. Operates in about 100 countries around the world.
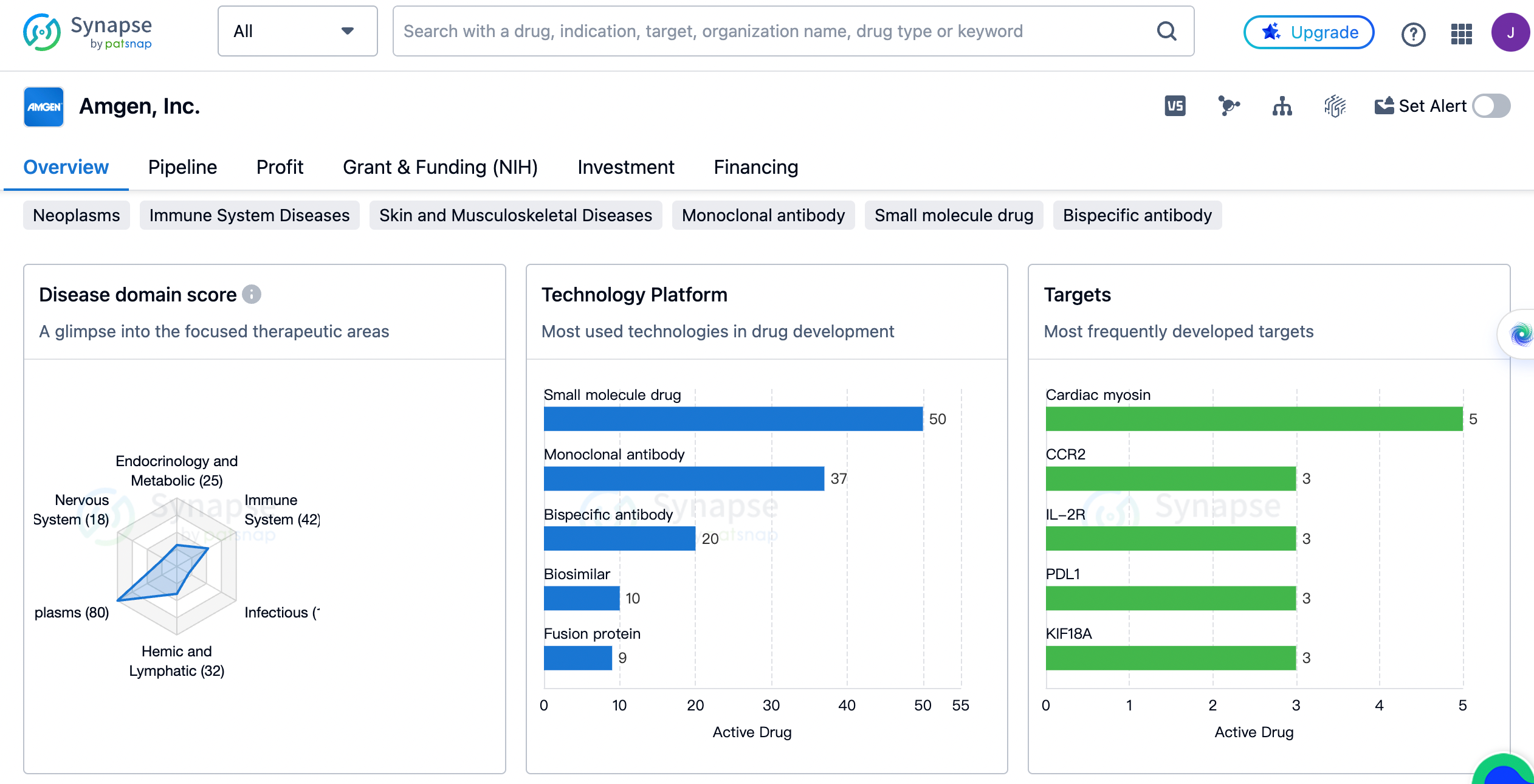
An overview of the distribution of therapeutic areas
The organization has focused on various therapeutic areas, with the highest number of drugs developed for neoplasms, totaling 80. This indicates Amgen's commitment to addressing the needs of cancer patients. Following neoplasms, the company has also developed drugs for skin and musculoskeletal diseases (45), immune system diseases (42), digestive system disorders (33), and hemic and lymphatic diseases (32). These therapeutic areas highlight Amgen's efforts to tackle a wide range of medical conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Amgen.
In addition to therapeutic areas, Table 2 highlights the most frequently developed targets by Amgen, Inc. and their corresponding drug count. Cardiac myosin, CCR2, IL-2R, PDL1, and KIF18A are the top targets, each with three drugs developed. This suggests that Amgen has invested significant resources in researching and developing drugs targeting these specific areas. Other targets with multiple drugs developed include PI3Kδ, PD-1, CD19 + CD3, TSLP, TNF-α, FLT3, 15-PGDH, CD3 + EGFR, CaSR, chemokine receptors, CD3 + PSMA, EPO receptor, proteasome, BCMA + CD3, and PRMT5.
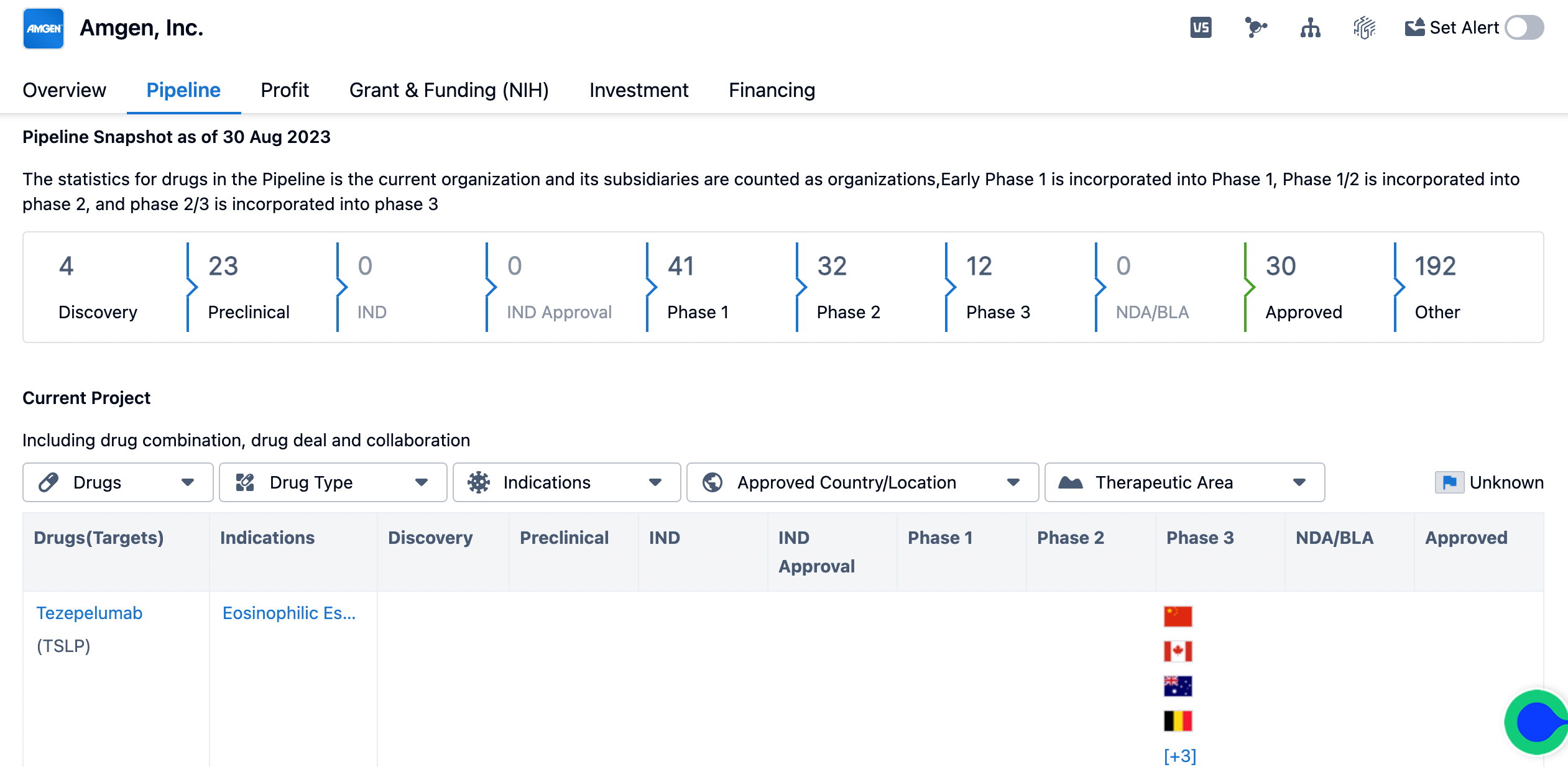
The pipeline of Amgen
Currently, Amgen has four drugs in the discovery phase and 23 drugs in the preclinical phase. The organization has a significant number of drugs in Phase 1 (41), Phase 2 (32), and Phase 3 (12) of clinical trials. This indicates that Amgen is actively progressing its drug candidates through the clinical trial process. The pipeline also shows that Amgen has 30 drugs that have been approved, indicating successful completion of the regulatory approval process. Additionally, there are 192 drugs categorized as "Other," which could include drugs in various stages of development or those that do not fit into the defined phases.
Amgen, Inc. has demonstrated a strong focus on developing drugs for a wide range of therapeutic areas, with a particular emphasis on neoplasms. This aligns with the growing need for effective treatments for cancer patients. The organization has also targeted specific molecules and receptors, such as cardiac myosin, CCR2, and IL-2R, indicating a strategic approach to drug development. The pipeline analysis reveals that Amgen has a robust portfolio of drugs in various stages of development. With a significant number of drugs in Phase 1, Phase 2, and Phase 3, the organization is actively advancing its candidates through clinical trials. This suggests a commitment to bringing innovative therapies to market. The approval of 30 drugs further demonstrates Amgen's success in obtaining regulatory clearance for its products.
Overall, Amgen, Inc. has established itself as a prominent player in the pharmaceutical industry, particularly in the field of biomedicine. The organization's focus on a diverse range of therapeutic areas and its extensive pipeline of drugs in various stages of development highlight its dedication to addressing unmet medical needs. As Amgen continues to progress its drug candidates through clinical trials and gain regulatory approvals, it is poised to make significant contributions to the healthcare industry and improve patient outcomes.