Analysis of Investment Trends and Licensing of Pharmaceuticals in the Healthcare Industry in January 2025
In January 2025, the global pharmaceutical industry's investment and licensing activities demonstrated a strong interest in novel treatment methods and technologies. From rare disease treatments to cardiovascular disease management, and groundbreaking advancements in oncology, this month’s collaborations encompassed various indications and innovative technologies. AB2 Bio's collaboration with Nippon Shinyaku focused on treating rare pediatric diseases with Tadekinig alfa, a novel recombinant human interleukin-18 binding protein (IL-18 BP), aimed at addressing high-inflammatory syndromes driven by IL-18. AbbVie and Neomorph partnered to develop molecular glue degraders, an innovative therapy targeting difficult-to-drug protein targets, bringing new hope to the fields of oncology and immunology. Additionally, Verve Therapeutics and Eli Lilly's collaboration is dedicated to utilizing gene-editing technology to treat cardiovascular diseases, particularly with VERVE-102, a drug capable of lowering levels of low-density lipoprotein cholesterol (LDL-C).
In these partnerships, a clear trend is observed: most of the drugs being licensed or invested in are at various stages of clinical trials, with some nearing the commercialization stage. For example, Tadekinig alfa is expected to enter the market soon, while VERVE-102 plans to announce its preliminary data readings in the second quarter of 2025.
1.AB2 Bio and Nippon Shinyaku advance Tadekinig Alfa treatment for rare pediatric diseases
On January 28, 2025, AB2 Bio reached an important option and licensing agreement with Nippon Shinyaku. According to this agreement, Nippon Shinyaku acquired exclusive rights to commercialize Tadekinig alfa in the United States. Tadekinig alfa is a novel recombinant human interleukin-18 binding protein (IL-18 BP), specifically designed to treat high-inflammatory syndromes driven by IL-18, a rare and severe pediatric disease that can lead to rapid multi-organ failure and death. This collaboration aims to accelerate the development and market introduction of this urgently needed treatment to meet the significant medical needs of children suffering from this rare disease and their families.
Under the terms of the agreement, AB2 Bio will initially receive up to $36 million in early payments, including $6 million paid at the time of signing. Additionally, AB2 Bio is eligible to receive up to $150 million in development and commercial milestone payments. Should Tadekinig alfa be successfully launched, AB2 Bio would also receive royalties from NS Pharma’s sales, with the potential total amount reaching up to $500 million. These funds provide necessary financial support for AB2 Bio and reflect both parties' commitment and confidence in advancing this innovative therapy.
AB2 Bio will continue to be responsible for preparing and submitting a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for approval of this indication. Once Nippon Shinyaku decides to exercise the option, and following BLA approval from AB2 Bio, its wholly-owned subsidiary NS Pharma will take over the marketing of Tadekinig alfa in the U.S. market. This marks significant collaboration between the two companies in advancing treatment for rare diseases and also signals hope for more patients affected by such conditions in the future.
2.AbbVie partners with Neomorph to venture into oncology and immunotherapy with a $1.64 billion collaboration to develop molecular glue degraders
On January 23, 2025, U.S.-based AbbVie and Neomorph, Inc. announced a significant collaboration and licensing option agreement to develop novel molecular glue degraders for use in the fields of oncology and immunology. This collaboration leverages AbbVie's extensive expertise in the development of oncology and immunotherapy drugs, combined with Neomorph’s leading advancements in its molecular glue discovery platform.
Molecular glue degraders are an innovative class of small molecule drugs designed to selectively target and trigger the degradation of proteins that cause cancer growth or disrupt immune system function, offering a more precise approach to treatment. These drugs have the potential to target proteins traditionally considered “undruggable,” opening new possibilities for treating hard-to-tackle diseases.
Under the terms of the agreement, Neomorph will receive an upfront payment from AbbVie and is eligible to receive up to $1.64 billion in option fees and milestone payments, as well as tiered royalties on net sales. This collaboration is significant as it marks a mutual commitment by both companies to explore and develop new treatment modalities and reflects their confidence in molecular glue degraders as an important component of the future pharmaceutical landscape. Through this partnership, AbbVie and Neomorph aim to launch innovative and effective therapies in the fields of oncology and immunology, thereby enhancing the quality of life for patients.
3.Verve Therapeutics teams up with Eli Lilly to advance cardiovascular disease treatments
On January 13, 2025, Verve Therapeutics and Eli Lilly announced a critical collaboration focused on developing gene-editing therapies for treating cardiovascular diseases. According to the agreement, Eli Lilly will make a $200 million upfront payment and a $50 million equity investment, with up to $350 million promised in milestone payments, bringing the potential total transaction value to approximately $600 million. This funding will support Verve’s R&D efforts, specifically for the VERVE-102 drug targeting the PCSK9 gene, which permanently lowers levels of low-density lipoprotein cholesterol (LDL-C) through a single intravenous injection.
The collaboration timeline sets several key milestones, including the anticipated release of preliminary data from the phase 1b Heart-2 clinical trial of VERVE-102 in the second quarter of 2025, along with an opportunity for Eli Lilly to opt into the PCSK9 project in the latter half of the year. Additionally, Verve plans to advance clinical trials for its ANGPTL3-targeting drug VERVE-201 and continue developing the VERVE-301 project targeting the LPA gene in collaboration with Eli Lilly. These steps signify a joint effort to provide innovative treatment solutions for patients with cholesterol-related cardiovascular diseases.
The purpose of this collaboration is to utilize Verve’s proprietary base editing technology and its GalNAc-LNP liver delivery system to develop a drug that can control cholesterol levels long-term or even for life with a single treatment. For Eli Lilly, this represents not only an opportunity to expand its cardiovascular disease treatment portfolio but also an acknowledgment of the potential of emerging gene editing technologies. From a broader perspective, this partnership aims to advance scientific frontiers and bring hope to hundreds of millions of cardiovascular disease patients worldwide, potentially significantly reducing the risk of heart attacks and strokes.
4. Araris Collaborates with Chugai Pharmaceutical to Develop Next-Generation Antibody-Drug Conjugates Using AraLinQ™ Technology
On January 9, 2025, Araris Biotech, a Swiss oncology biotech company specializing in next-generation Antibody-Drug Conjugates (ADCs), entered into a significant Research Collaboration and Option (RCO) agreement with Chugai Pharmaceutical, a member of the Roche Group. Under this agreement, Araris will utilize its proprietary linker conjugation platform, AraLinQ™, along with Chugai’s antibodies targeting undisclosed markers, to develop innovative ADC therapeutics. This collaboration leverages Araris’s technological edge—its ability to efficiently conjugate payloads to antibodies without pre-modification—aiming to develop next-generation ADCs with enhanced efficacy and tolerability.
Financial terms of the partnership indicate that Chugai Pharmaceutical will pay an undisclosed upfront fee and fund all research activities. After Chugai exercises its option rights on the development results, it will be responsible for all subsequent development, manufacturing, and global commercialization activities. Should certain developmental, regulatory, and commercial milestones be met, Araris could receive potential milestone payments of up to $780 million, in addition to royalties on the net sales of the products.
The purpose of this collaboration is to advance ADC technology, particularly through Araris’s innovative AraLinQ platform, which allows efficient and precise attachment of various payloads to a single antibody without altering its structure, offering safer and more effective solutions for cancer treatment. Araris envisions creating a world free from traditional chemotherapy with this 'smart missile' approach to overcome long-term challenges like cancer resistance. For Chugai Pharmaceutical, this represents not only an opportunity to expand its oncology pipeline but also reflects its commitment to discovering the most promising next-generation cancer therapies. This cooperation could lead the medical community towards a new trend in treatment, offering more precise and personalized therapy options for patients.
5. Atavistik Bio and Pfizer Partner to Explore Novel Allosteric Modulators Using AMPS™ Platform
On January 3, 2025, Atavistik Bio announced a significant research collaboration with Pfizer, utilizing Atavistik Bio’s proprietary AMPS™ platform to accelerate the discovery of novel precision allosteric therapies to address significant unmet medical needs. As per the collaboration agreement, Atavistik Bio will conduct research and development on novel allosteric modulators targeting two undisclosed markers selected by Pfizer. Upon completion of the research phase, Pfizer will have the option to license these projects, although specific financial terms have not been disclosed.
The core of this cooperation is the AMPS™ platform of Atavistik Bio, an integrated discovery engine capable of rapidly identifying functional cryptic pockets across a broad range of targets, and designing small molecule drugs aimed at traditionally challenging drug targets. Atavistik Bio is advancing its precision oncology small molecule therapy pipeline, including ATV-1601, a selective allosteric inhibitor planned for early clinical trials in 2025 for solid tumors. This collaboration not only helps expand the scope of Atavistik Bio’s technology application but could also introduce new therapeutic solutions in oncology, particularly for patient groups who respond poorly to current treatments.
This collaborative effort combines Atavistik Bio’s innovative capabilities in the discovery of allosteric drugs with Pfizer’s global resources and market influence in the pharmaceutical industry. Through this partnership model, both parties aim to develop new drugs with higher selectivity, better tolerability, and more effective therapeutic outcomes more efficiently. Moreover, this marks a joint commitment by both companies to explore next-generation cancer therapies, potentially driving the industry forward and offering more treatment options to patients worldwide.
Summary
From the analysis of the investment and licensing activities in the pharmaceutical sector in January 2025, we have identified a trend: investors and pharmaceutical companies are increasingly inclined to support therapies that are highly innovative and have the potential for significant impact. Whether it’s precision treatment plans for rare diseases or innovative therapies for major public health challenges such as cardiovascular diseases and cancer, the collaborations this month reflect this direction. It is noteworthy that many drugs being invested in or licensed are in critical stages of clinical development, which means that, in the coming years, we will see more new drugs coming to the market based on these innovative technologies.
Looking ahead, as technological advancements and scientific understanding deepen, we foresee more funding flowing into research and development projects that can offer more efficient and safer treatment options. Additionally, international collaboration will continue to be a key force driving the development of the pharmaceutical industry, especially in overcoming geographical limits and technological barriers. For investors, identifying and supporting companies that can bring transformative changes in areas of unmet medical needs will be one of the key strategies for realizing long-term value.
In conclusion, the investment and licensing dynamics at the beginning of 2025 reveal that the pharmaceutical industry is moving towards more personalized and precise developments. With the emergence of more innovative therapies and deeper international collaboration, we have reasons to believe that future healthcare will become more effective, greatly improving the quality of life for patients.
How to get the latest progress on drug deals?
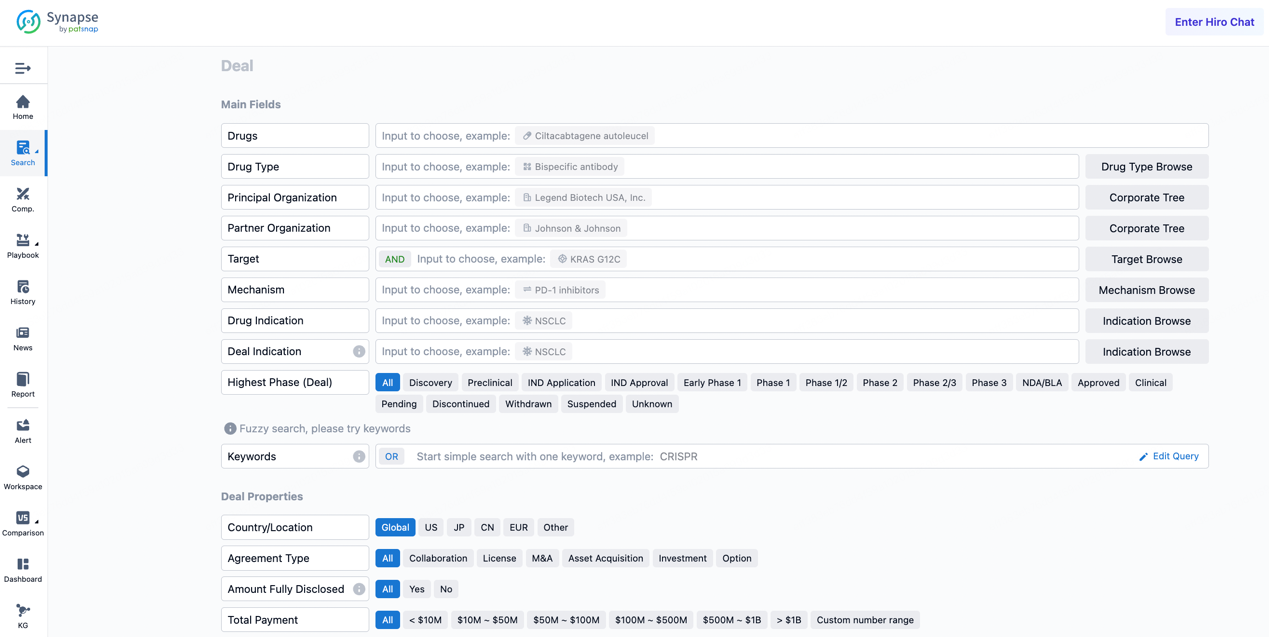
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
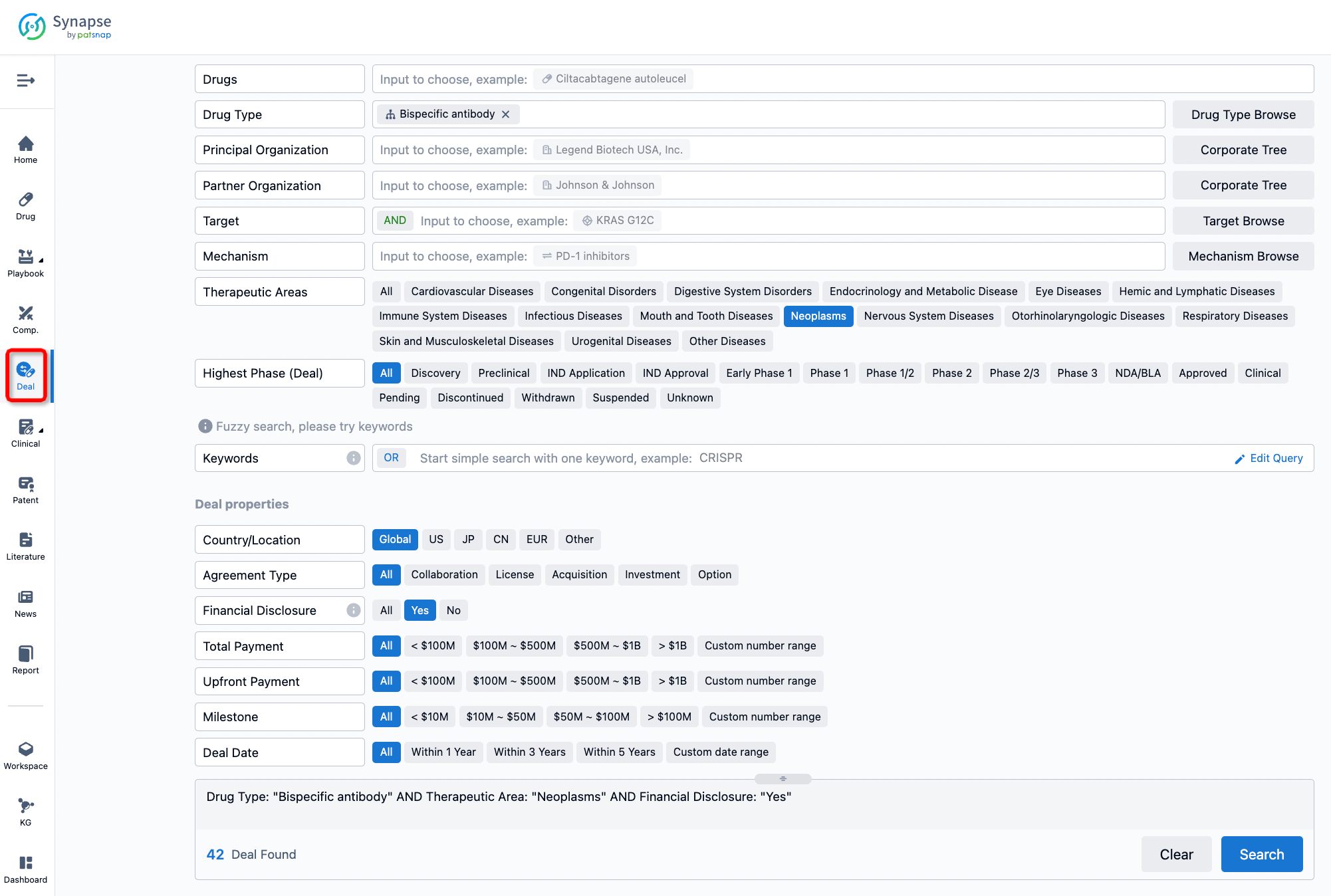
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
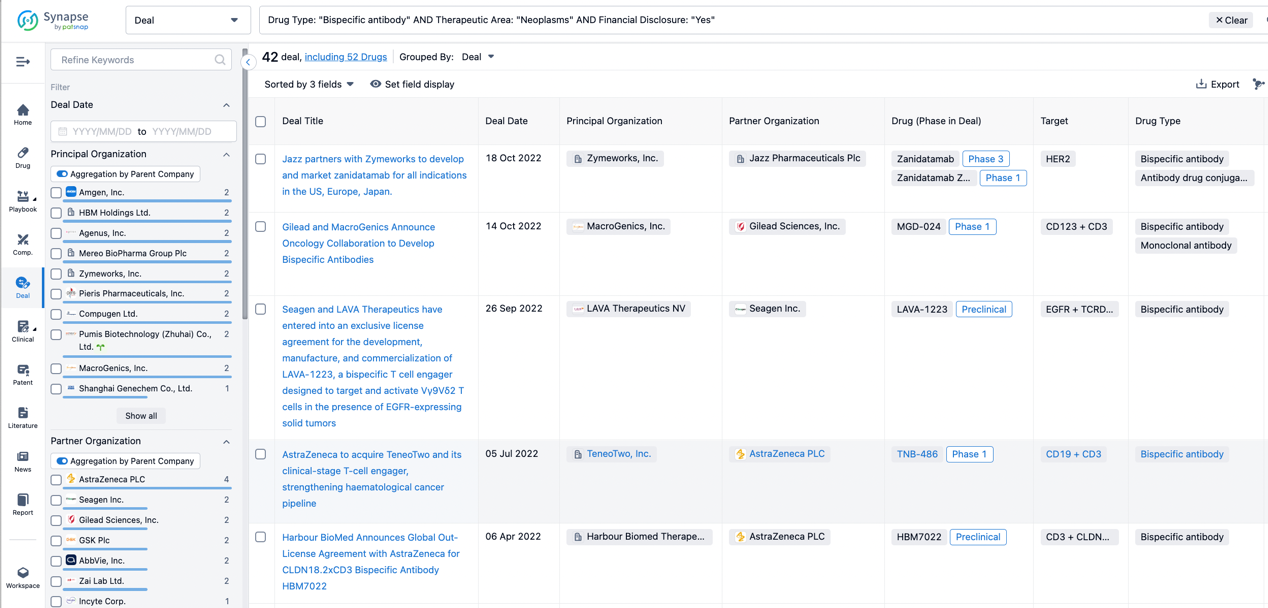
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
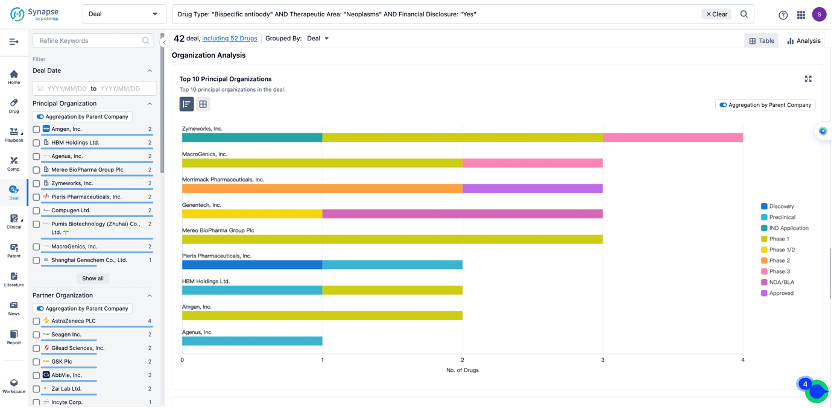
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
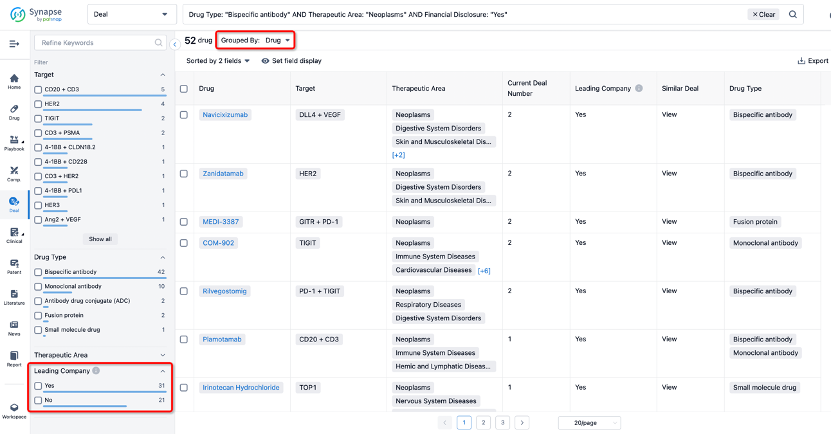
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
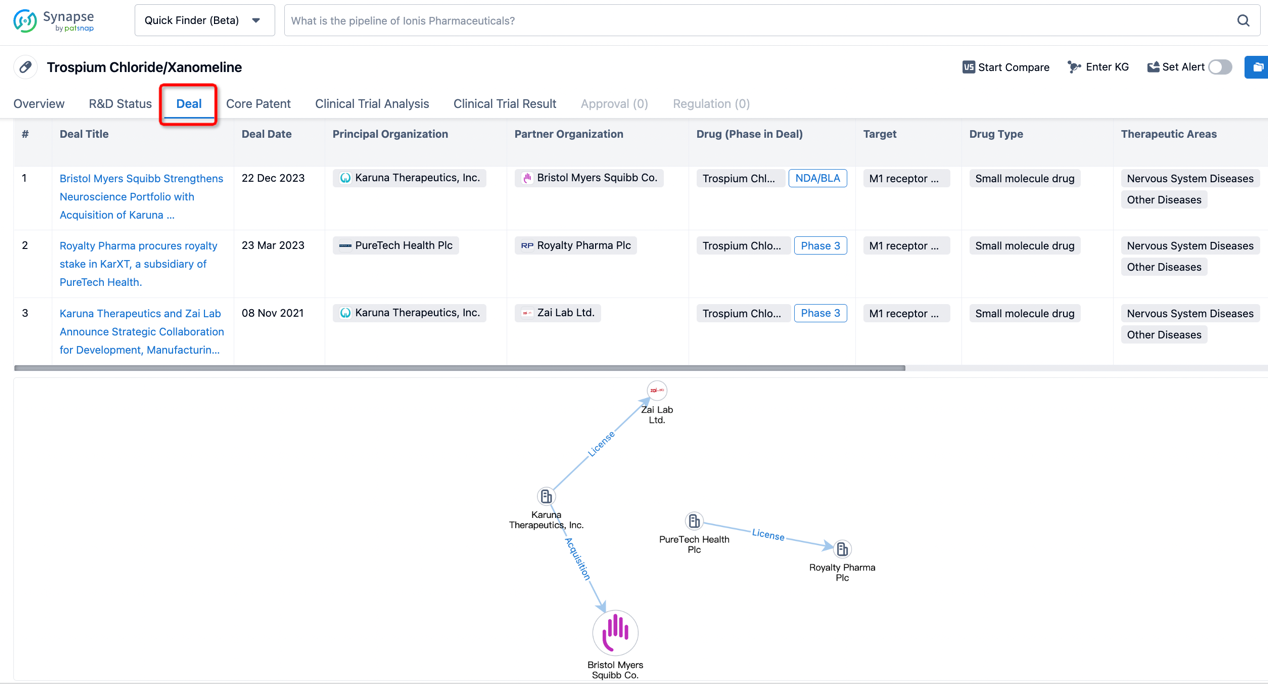
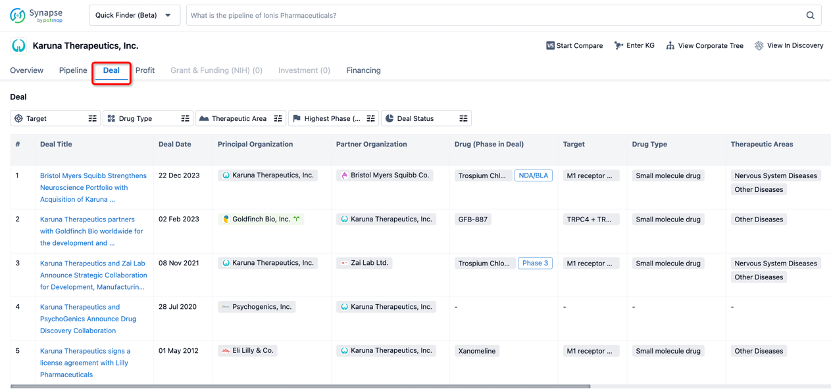
Click on the image below to explore new pharmaceutical funding transactions!