Analysis on the Clinical Research Progress of major histocompatibility complex class II
Major histocompatibility complex class II (MHC II) molecules play a crucial role in the immune system by presenting antigens to CD4+ T cells. These molecules are expressed on the surface of antigen-presenting cells, such as macrophages, dendritic cells, and B cells. MHC II molecules bind to antigens derived from pathogens and present them to CD4+ T cells, initiating an immune response. This interaction is essential for the activation of helper T cells, which coordinate the immune response by releasing cytokines and activating other immune cells. MHC II molecules are vital for the recognition and elimination of foreign invaders, contributing to the body's defense against infections and diseases.
MAJOR HISTOCOMPATIBILITY COMPLEX Competitive Landscape
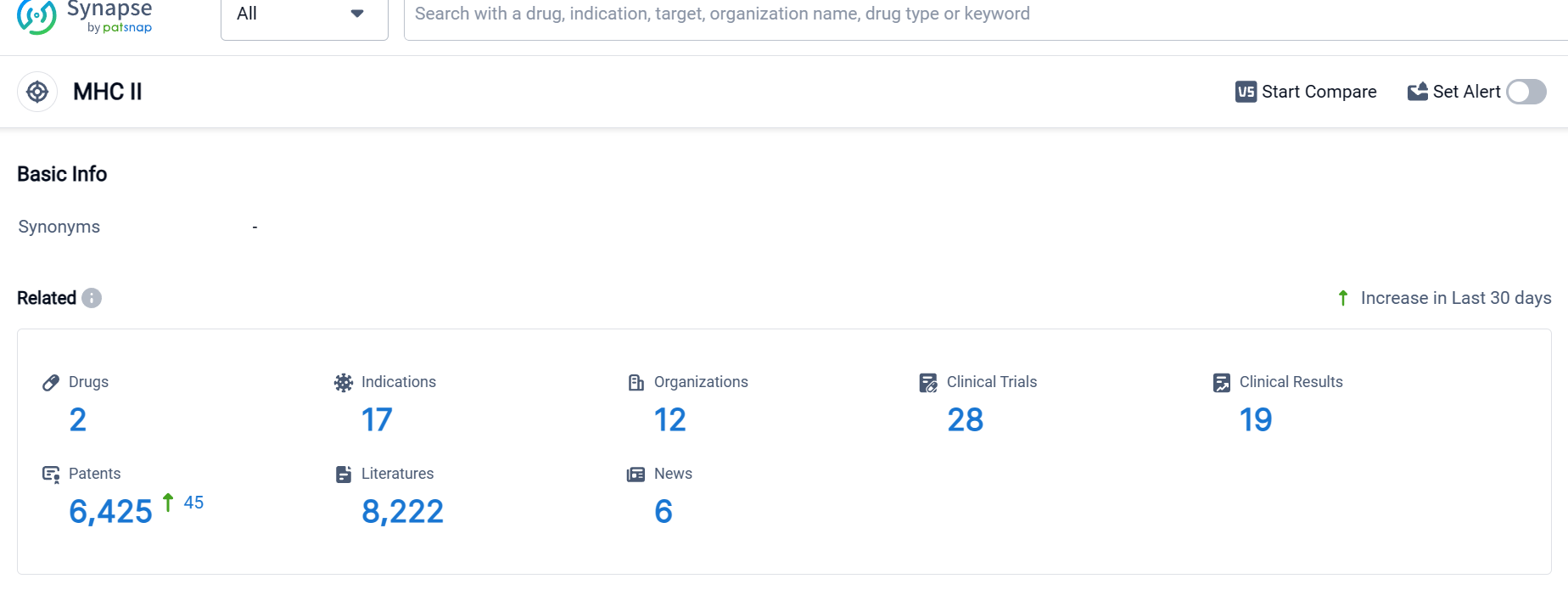
According to Patsnap Synapse, as of 6 Oct 2023, there are a total of 2 MHC II drugs worldwide, from 12 organizations, covering 17 indications, and conducting 28 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target MHC II reveals a competitive landscape with multiple companies focusing on its development. Immutep Ltd., Worg Pharmaceuticals, and Merck KGaA are among the companies with the highest stage of development. The indications for drugs targeting MHC II cover a wide range of diseases, indicating their potential therapeutic applications. Fusion proteins, therapeutic vaccines, and combination vaccines are the drug types progressing most rapidly under the current target. The presence of biosimilars suggests intense competition in the market. China has shown progress in the development of drugs targeting MHC II, indicating its growing role in the pharmaceutical industry. Overall, the target MHC II presents opportunities for innovation and competition in the pharmaceutical industry, with potential benefits for various disease indications.
Key drug:Eftilagimod alpha
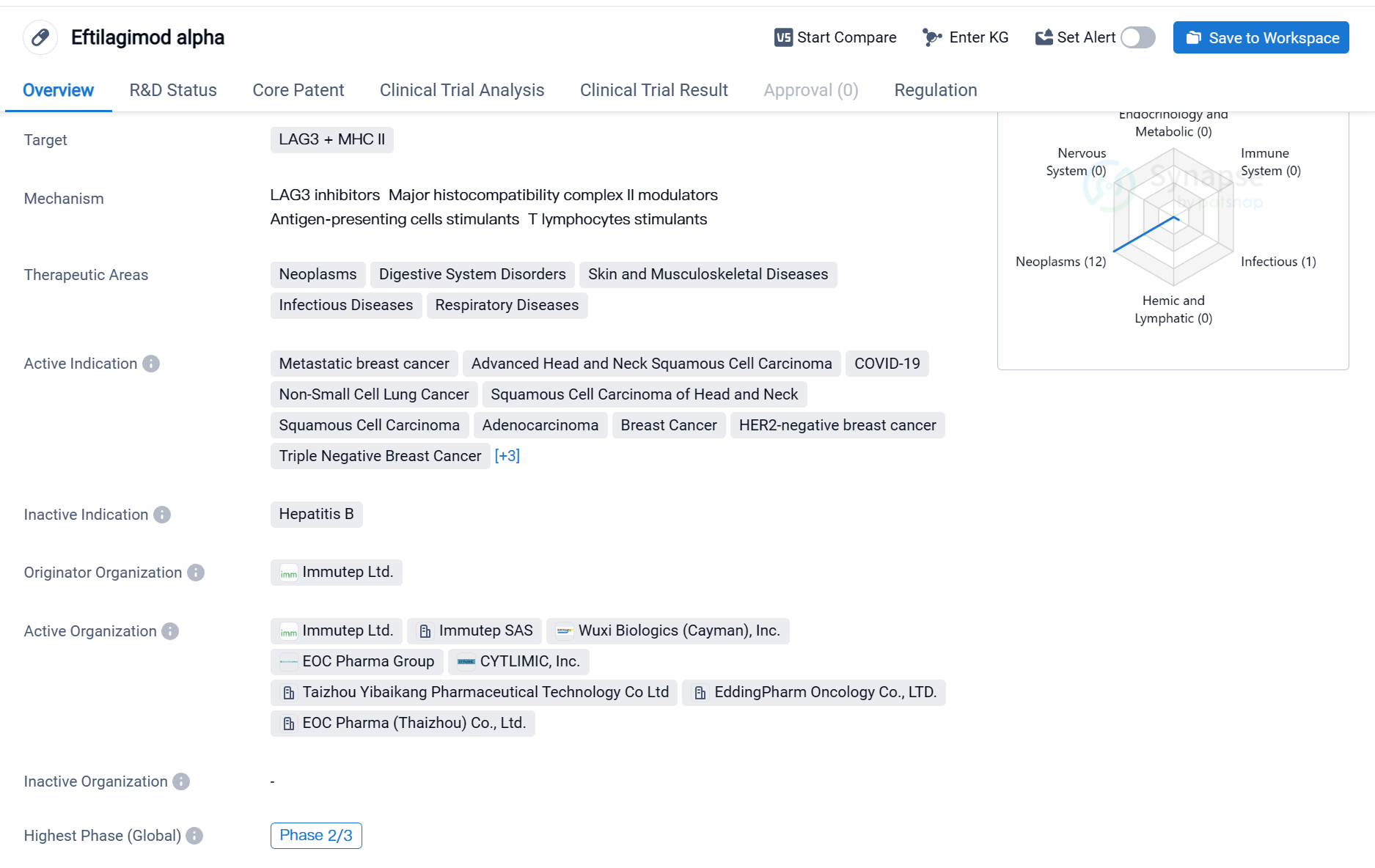
Eftilagimod alpha is a fusion protein drug that targets LAG3 (lymphocyte-activation gene 3) and MHC II (major histocompatibility complex class II). It is being developed by Immutep Ltd., an originator organization in the pharmaceutical industry. The drug is currently in the highest phase of clinical development, which is Phase 2/3 globally and Phase 2 in China.
Eftilagimod alpha has shown potential therapeutic applications in various therapeutic areas, including neoplasms (abnormal growth of cells), digestive system disorders, skin and musculoskeletal diseases, infectious diseases, and respiratory diseases. It has been specifically indicated for the treatment of metastatic breast cancer, advanced head and neck squamous cell carcinoma, COVID-19, non-small cell lung cancer, squamous cell carcinoma of the head and neck, squamous cell carcinoma, adenocarcinoma, breast cancer, HER2-negative breast cancer, triple-negative breast cancer, liver cancer, solid tumors, and melanoma.
The drug has received Fast Track designation, indicating that it is being expedited through the regulatory process due to its potential to address unmet medical needs. This designation is granted by regulatory authorities to accelerate the development and review of drugs that demonstrate promising therapeutic benefits for serious or life-threatening conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In summary, Eftilagimod alpha is a fusion protein drug developed by Immutep Ltd. that targets LAG3 and MHC II. It is currently in Phase 2/3 globally and Phase 2 in China. The drug has shown potential in treating various diseases, including neoplasms, digestive system disorders, skin and musculoskeletal diseases, infectious diseases, and respiratory diseases. It has received Fast Track designation, highlighting its potential to address unmet medical needs.