Analysis on the Clinical Research Progress of Mineralocorticoid Receptor Antagonist
Mineralocorticoids are steroid hormones secreted by the cells of the zona glomerulosa of the adrenal cortex. Their primary physiological function is to maintain the balance of water and electrolytes in the human body.
The role of the MR (Mineralocorticoid Receptor) in the human body is crucial for maintaining electrolyte and fluid balance. MR is a type of receptor found in various tissues, including the kidneys, heart, and brain. When activated by the hormone aldosterone, MR regulates the reabsorption of sodium and water in the kidneys, leading to increased blood volume and blood pressure. This receptor also plays a role in regulating potassium levels and maintaining the balance of electrolytes. Dysfunction of the MR can lead to conditions such as hypertension, electrolyte imbalances, and cardiovascular diseases. Understanding the role of MR is essential for developing targeted therapies in the pharmaceutical industry.
MINERALOCORTICOID RECEPTOR Competitive Landscape
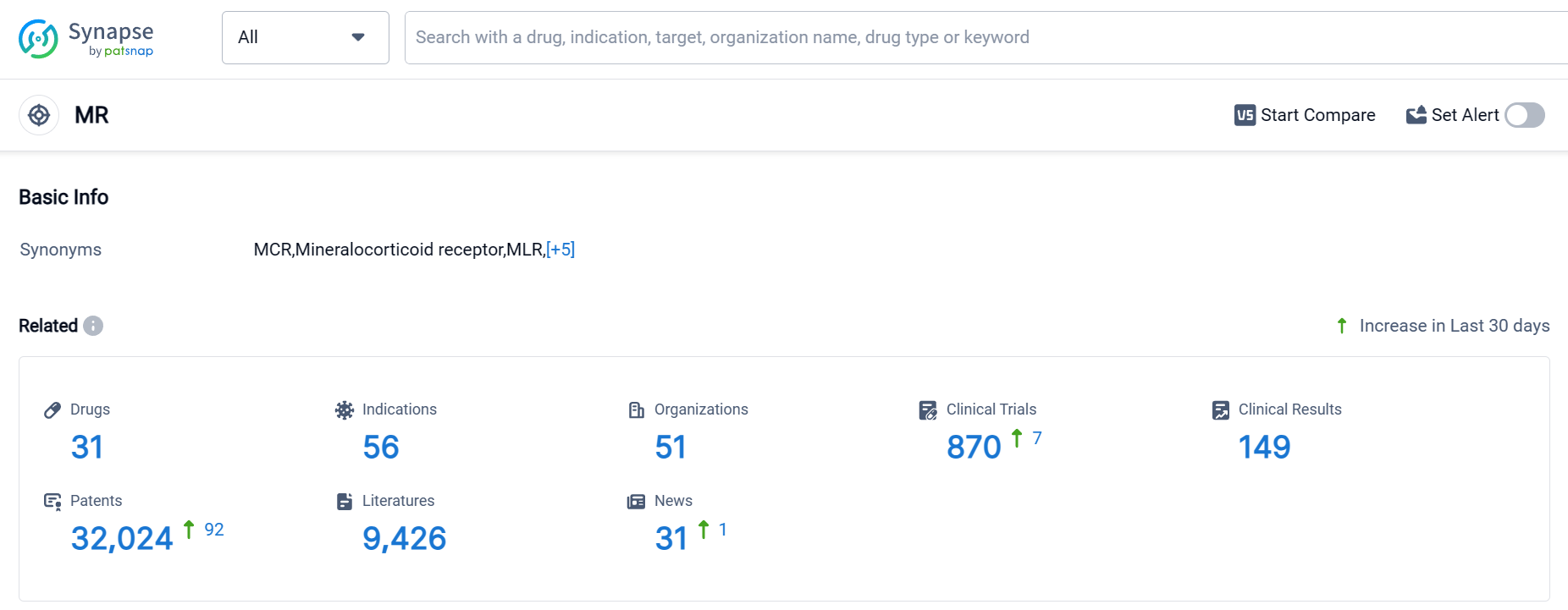
According to Patsnap Synapse, as of 1 Oct 2023, there are a total of 31 MR drugs worldwide, from 51 organizations, covering 56 indications, and conducting 870 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Based on the analysis of the provided data, the current competitive landscape of the target MR is characterized by the involvement of multiple companies, with Bayer AG and Pfizer Inc. leading in terms of drug approvals. The most common approved indications include hypertension, contraception, and heart failure. Small molecule drugs dominate the development phases, indicating their effectiveness and market potential. The United States, European Union, Japan, and China are the key countries driving development under the target MR.
To gain a more comprehensive understanding of the competitive landscape and future development, further analysis beyond the provided data is recommended. This may include examining additional companies, indications, drug types, and countries/locations involved in the target MR. Additionally, monitoring the progress of biosimilars and their impact on the innovative drug market is crucial. Overall, the target MR presents opportunities for growth and innovation in the pharmaceutical industry.
The world's first non-steroidal selective mineralocorticoid receptor antagonist:Finerenone
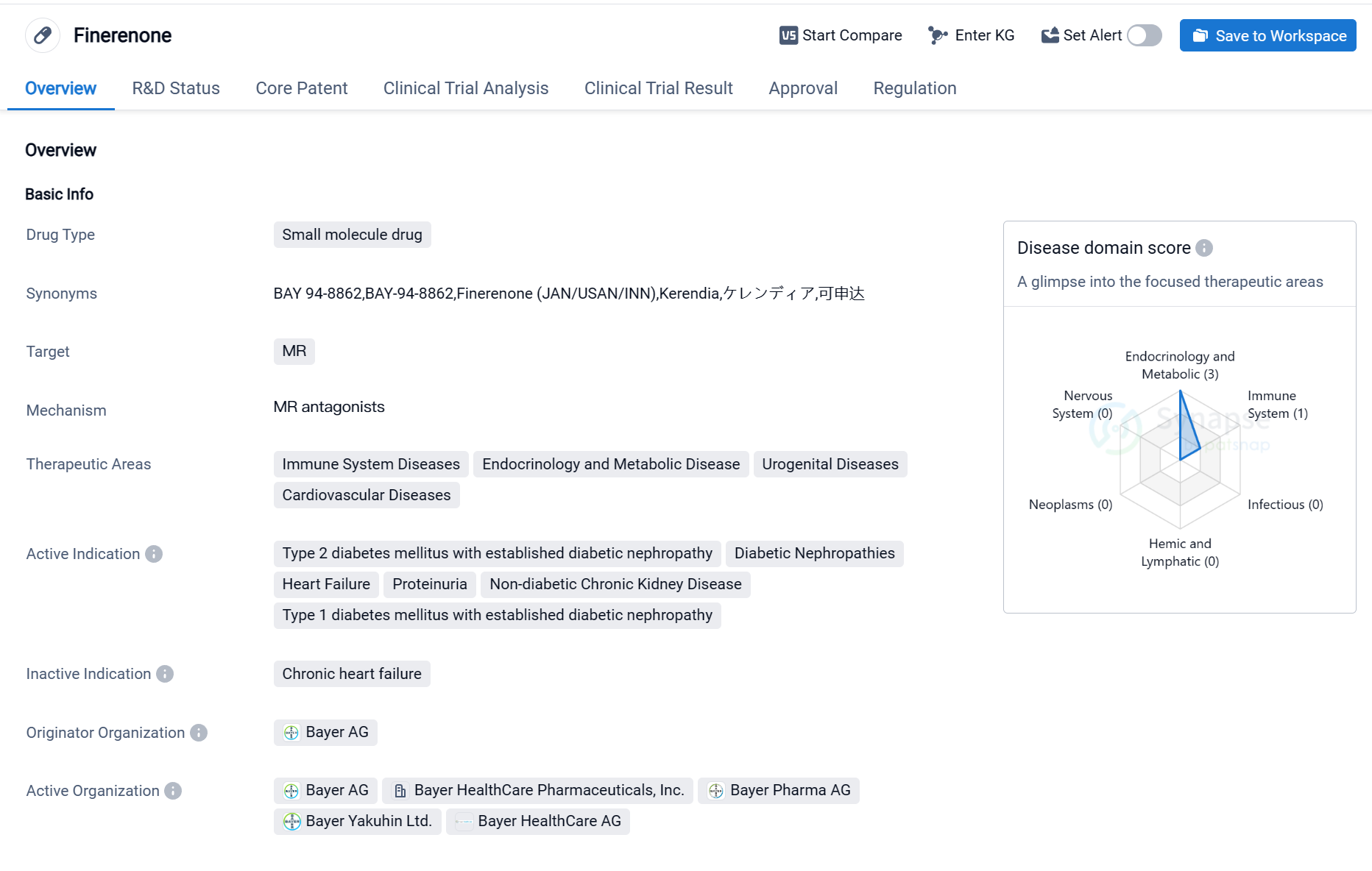
Finerenone is a small molecule drug that targets the mineralocorticoid receptor (MR). It is primarily used in the treatment of various diseases related to the immune system, endocrinology and metabolic disorders, urogenital diseases, and cardiovascular diseases. The drug has been approved for the treatment of type 2 diabetes mellitus with established diabetic nephropathy, diabetic nephropathies, heart failure, proteinuria, non-diabetic chronic kidney disease, and type 1 diabetes mellitus with established diabetic nephropathy.
Finerenone was developed by Bayer AG, a leading pharmaceutical company. It has received approval for its highest phase in the global market. The drug obtained its first approval in the United States in July 2021. The regulatory status of Finerenone includes being designated as a Fast Track drug, which expedites the development and review process, and it is also part of a Special Review Project.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As a small molecule drug, Finerenone acts by targeting the MR, a receptor involved in regulating salt and water balance in the body. By modulating the MR, Finerenone can potentially improve outcomes in patients with various diseases, including those related to the kidneys and cardiovascular system.
The approval of Finerenone represents a significant advancement in the field of biomedicine, particularly in the treatment of diabetes-related complications and cardiovascular diseases. Its approval for multiple indications highlights its potential to address unmet medical needs in patients with diverse conditions.
In summary, Finerenone is a small molecule drug developed by Bayer AG that targets the MR. It has been approved for the treatment of various diseases, including type 2 diabetes mellitus with established diabetic nephropathy, heart failure, and proteinuria. The drug has received its highest phase approval globally and in China, with its first approval in the United States. The regulatory status of Finerenone includes Fast Track designation and participation in a Special Review Project. Its approval signifies a significant advancement in the field of biomedicine and offers potential benefits to patients with different medical conditions.