Apogee Initiates Phase 2 Trial of APG777 in Atopic Dermatitis Treatment
Apogee Therapeutics, Inc., a biotechnology firm in the clinical-stage focusing on the development of unique biologics for treating atopic dermatitis, chronic obstructive pulmonary disease, asthma, and various inflammatory and immunological disorders, has announced the commencement of dosing for its Phase 2 trial of APG777 in individuals suffering from moderate-to-severe atopic dermatitis.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
APG777 is an innovative, subcutaneously-administered, half-life extended monoclonal antibody that targets IL-13—a pivotal cytokine in inflammation and a key factor in atopic dermatitis (AD).
"We are excited to commence patient dosing in our Phase 2 study of APG777, marking a pivotal milestone in the progression of this program and bringing us closer to understanding the impact a fully-optimized, half-life extended antibody could have on AD treatment compared to existing biologics," stated Michael Henderson, MD, CEO of Apogee.
"The launch of this Phase 2 study is backed by the highly promising results from our Phase 1 trial involving healthy volunteers, reported earlier this year. With what we believe to be a potentially best-in-class pharmacokinetic profile, consistent pharmacodynamic responses, and a favorable safety profile, APG777 could provide enhanced clinical outcomes with less frequent dosing compared to current standards of care. We eagerly anticipate the trial's progression and the release of initial 16-week data in the latter half of 2025," added Michael Henderson.
The Phase 2 clinical trial for APG777 is a randomized, placebo-controlled, 16-week study involving patients with moderate-to-severe AD. Designed to merge the typical Phase 2a and 2b segments into a single protocol, Part A is expected to enroll roughly 110 patients randomized in a 2:1 ratio to receive APG777 or placebo. Patients on APG777 will receive an induction regimen of 720mg at weeks 0 and 2, followed by 360mg at weeks 4 and 12.
Patients who respond to treatment will proceed to APG777 maintenance, assessing dosing intervals of 3 to 6 months. Part B will involve a placebo-controlled dose optimization with around 360 patients randomized in a 1:1:1:1 ratio to high, medium, or low doses of APG777 versus placebo. The primary endpoint for each part of the study is the mean percentage change in EASI score from baseline to Week 16.
In comparative preclinical studies, APG777 showed equal or superior potency to lebrikizumab in inhibiting IL-13 signaling. Given its potentially best-in-class pharmacokinetic profile, APG777 may offer enhanced clinical responses due to higher drug exposure during induction, while allowing for infrequent dosing as seldom as once every three or six months. AD is a chronic inflammatory skin condition that can cause sleep disturbances, psychological stress, increased infection risk, and chronic pain, significantly affecting quality of life.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
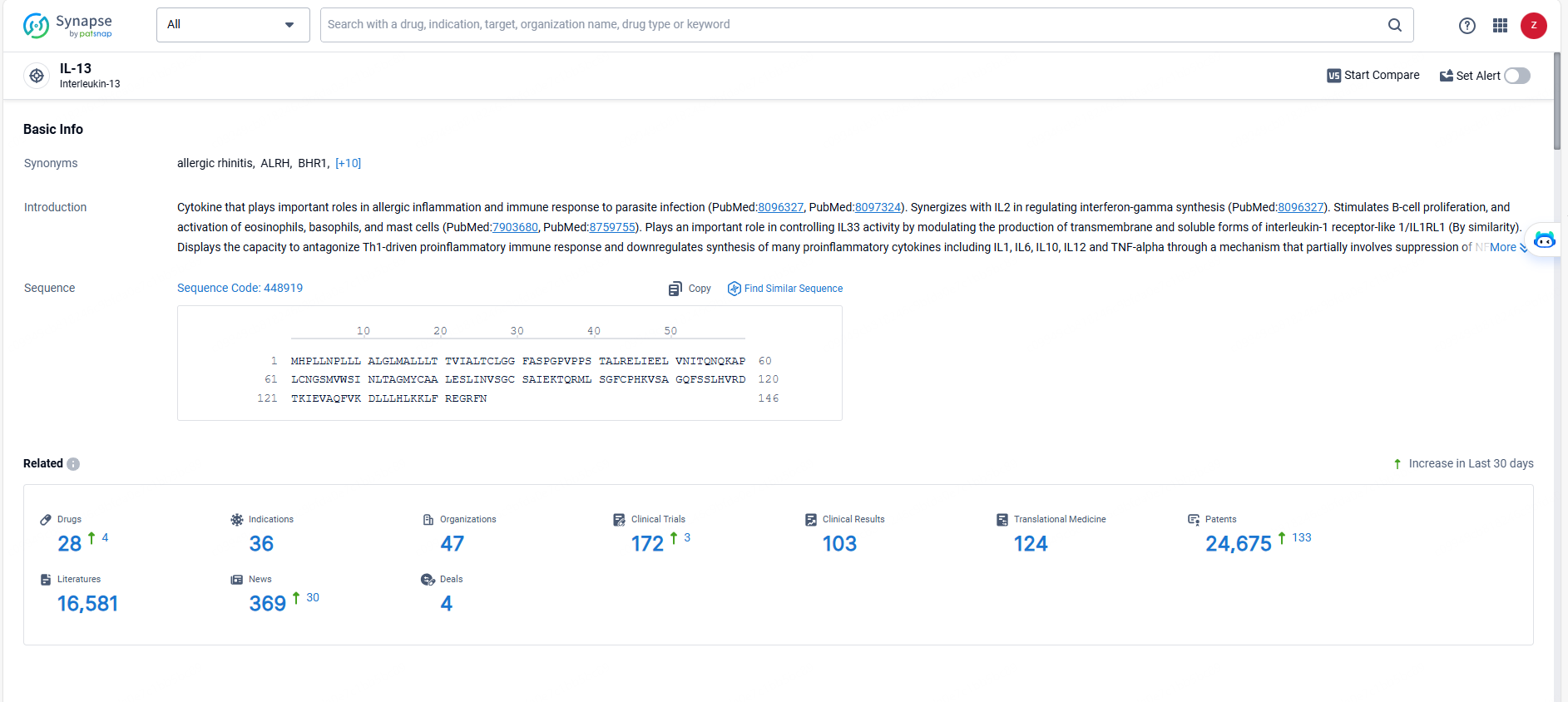
According to the data provided by the Synapse Database, As of May 23, 2024, there are 28 investigational drugs for the IL-13 targets, including 36 indications, 47 R&D institutions involved, with related clinical trials reaching 172, and as many as 24675 patents.
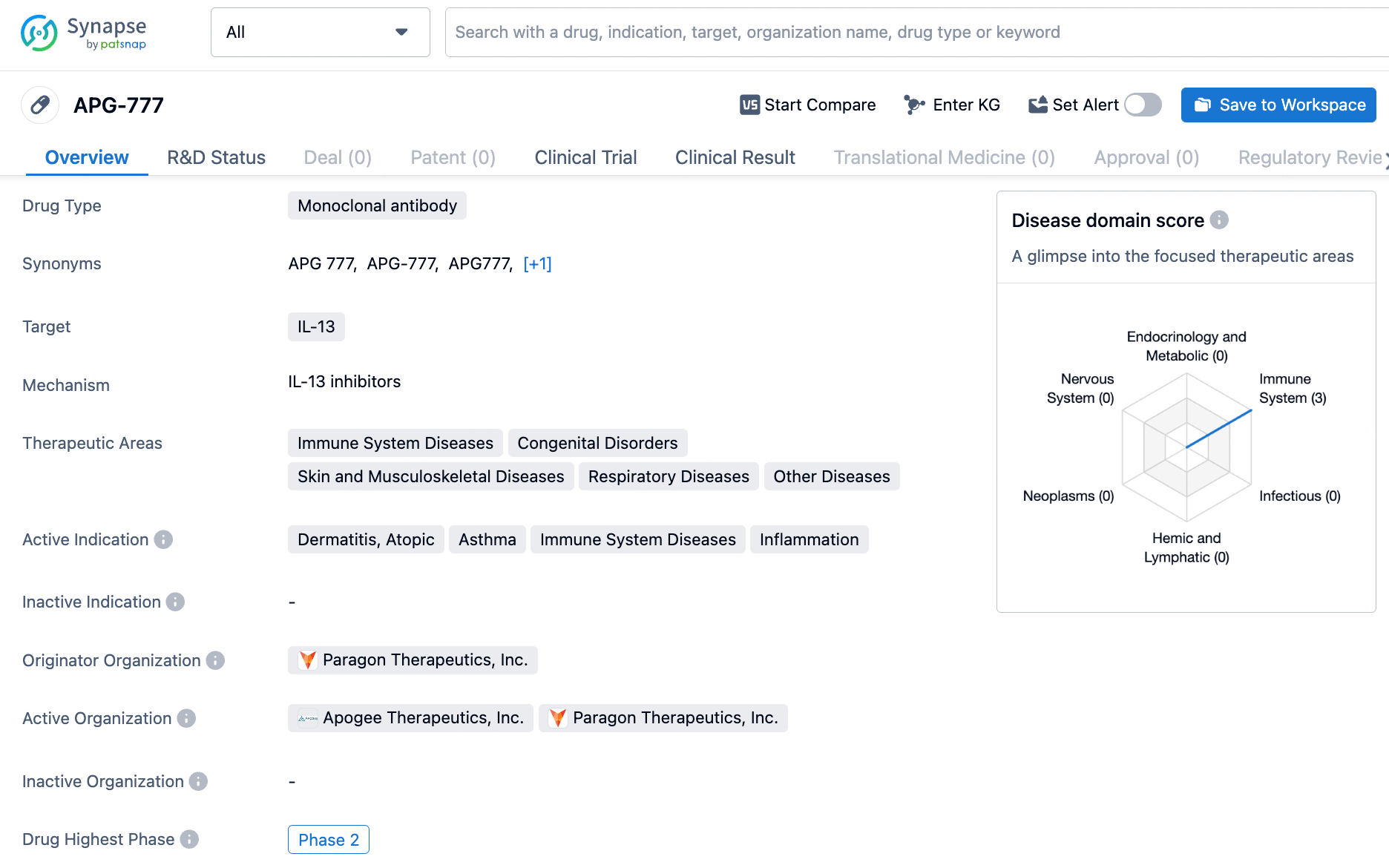
APG-777 is a monoclonal antibody drug targeting IL-13, with a focus on treating immune system diseases, congenital disorders, skin and musculoskeletal diseases, respiratory diseases, and other diseases. Its active indications include dermatitis, atopic, asthma, immune system diseases, and inflammation. The drug is currently in Phase 2 of development, and its progress reflects the ongoing efforts in the pharmaceutical industry to bring innovative therapies to patients in need.