Ractigen Therapeutics Gets NMPA Approval for Phase 1 Trials of RAG-17 in Chinese ALS Patients
Ractigen Therapeutics, a pharmaceutical company dedicated to the development of innovative treatments, has revealed that the Center for Drug Evaluation under China’s National Medical Products Administration has given the green light to their Investigational New Drug application. This approval paves the way for the commencement of Phase 1 clinical trials in China for RAG-17, which is aimed at treating Amyotrophic Lateral Sclerosis.
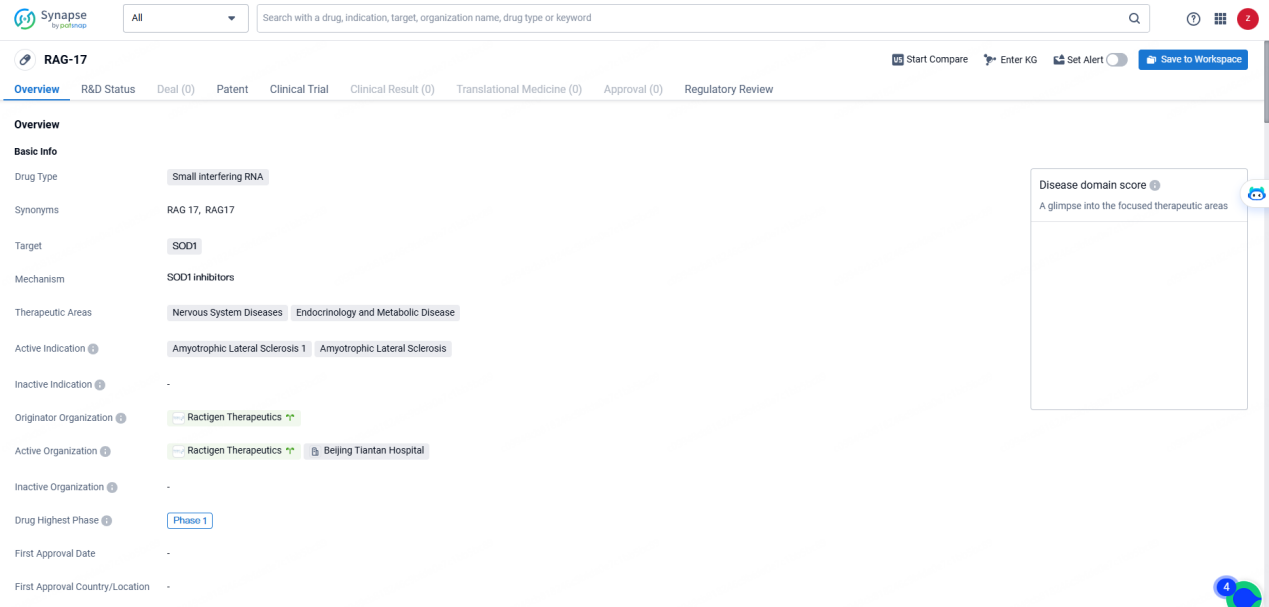
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The Phase I study initiating the IND is a randomized, double-blind, placebo-controlled trial assessing the safety, tolerability, pharmacokinetics, and pharmacodynamics of RAG-17 in ALS patients harboring the Superoxide Dismutase 1 (SOD1) mutation.
Dr. Long-Cheng Li, the Founder, President, and CEO of Ractigen, expressed excitement about this pivotal achievement: “This moment is crucial for our company, as RAG-17 is our first siRNA drug targeting the central nervous system to gain CDE approval. We look forward to advancing this therapy into clinical trials for ALS patients and are hopeful about its potential to provide substantial benefits to those with the SOD1 mutation.”
Mr. Lei Cai, an eminent ALS advocate in China who also battles the disease, shared his hopeful outlook regarding RAG-17: “I am very optimistic about RAG-17. Its innovative approach and promising early results give me great confidence in its potential. I believe this drug could offer significant hope and tangible benefits to the ALS community, particularly for those with the SOD1 mutation.”
In March 2023, RAG-17 received Orphan Drug Designation from the U.S. Food and Drug Administration and has been authorized for clinical trials in the United States. Additionally, an Investigator-Initiated Trial is currently ongoing for RAG-17, with 6 patients enrolled and treated, demonstrating encouraging preliminary safety and efficacy outcomes.
RAG-17 is a therapeutic siRNA specifically designed to inhibit the SOD1 gene in ALS patients with pathogenic mutations. RAG-17 leverages Ractigen’s proprietary SCAD™ delivery platform, combining siRNA with an accessory oligonucleotide to enhance its effectiveness in central nervous system tissues.
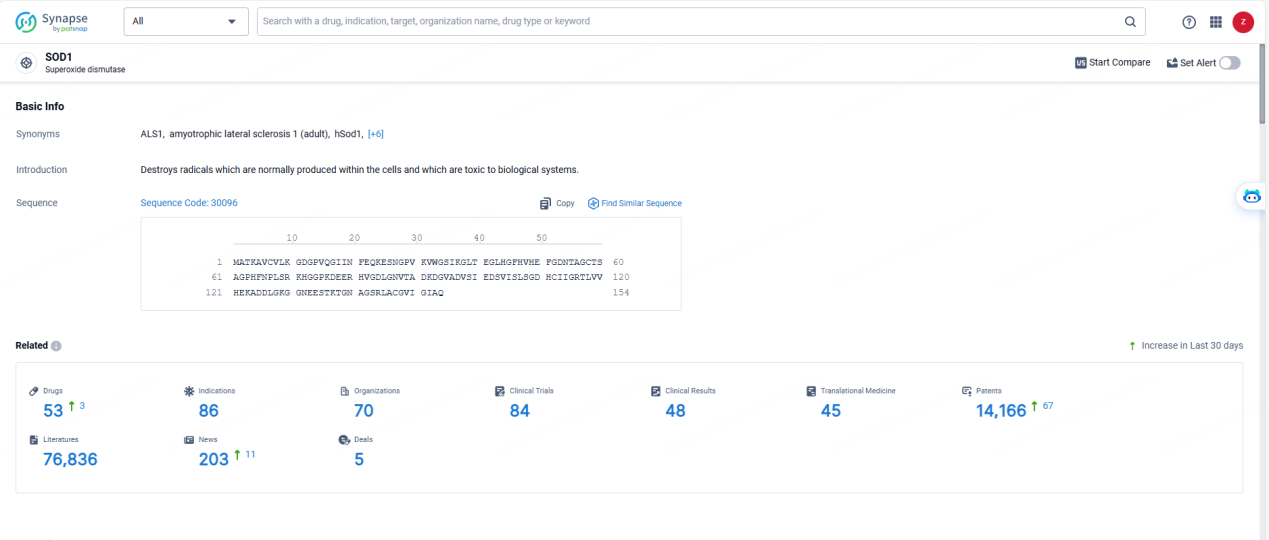
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 23, 2024, there are 53 investigational drugs for the SOD1 targets, including 86 indications, 70 R&D institutions involved, with related clinical trials reaching 84, and as many as 14166 patents.
RAG-17 targets SOD1 and focusing on therapeutic areas such as Nervous System Diseases and Endocrinology and Metabolic Disease. It has reached Phase 1 of development globally and Early Phase 1 in China, with a regulatory status as an Orphan Drug. The drug's focus on addressing ALS and related conditions through its targeting of SOD1 represents a significant development in the pharmaceutical industry's efforts to address unmet medical needs in the field of bio.