Voyager Therapeutics Administers Initial Doses in Alzheimer’s Drug Trial VY-TAU01
Voyager Therapeutics, Inc., a biotech firm focused on the advancement of neurogenetic therapies, revealed that the initial subjects have been administered doses in a Phase 1a single ascending dose study of VY-TAU01. This investigational anti-tau antibody is designed to prevent the proliferation of pathological tau associated with Alzheimer’s disease.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The purpose of the randomized, double-blind, placebo-controlled, single ascending dose (SAD) trial is to assess the safety profile and pharmacokinetic characteristics of VY-TAU01 in healthy adult participants. This research is taking place at one location in the United States, with an expected enrollment of around 48 individuals across various cohorts.
Voyager foresees that the outcomes of the SAD trial will guide the planning of a Phase 1b multiple ascending dose (MAD) trial in patients with early-stage Alzheimer’s disease, projected to commence in 2025. There is potential for the MAD study to produce preliminary tau PET imaging data by the latter half of 2026, which could demonstrate whether VY-TAU01 effectively slows the propagation of pathological tau in the brain.
“The commencement of VY-TAU01’s clinical development for Alzheimer’s disease marks a significant achievement for Voyager. This progress showcases our neurology drug development team's capabilities, which are crucial as we advance three independently-developed and partnered gene therapies in neurology towards IND submissions next year,” stated Toby Ferguson, M.D., Ph.D., Chief Medical Officer of Voyager Therapeutics.
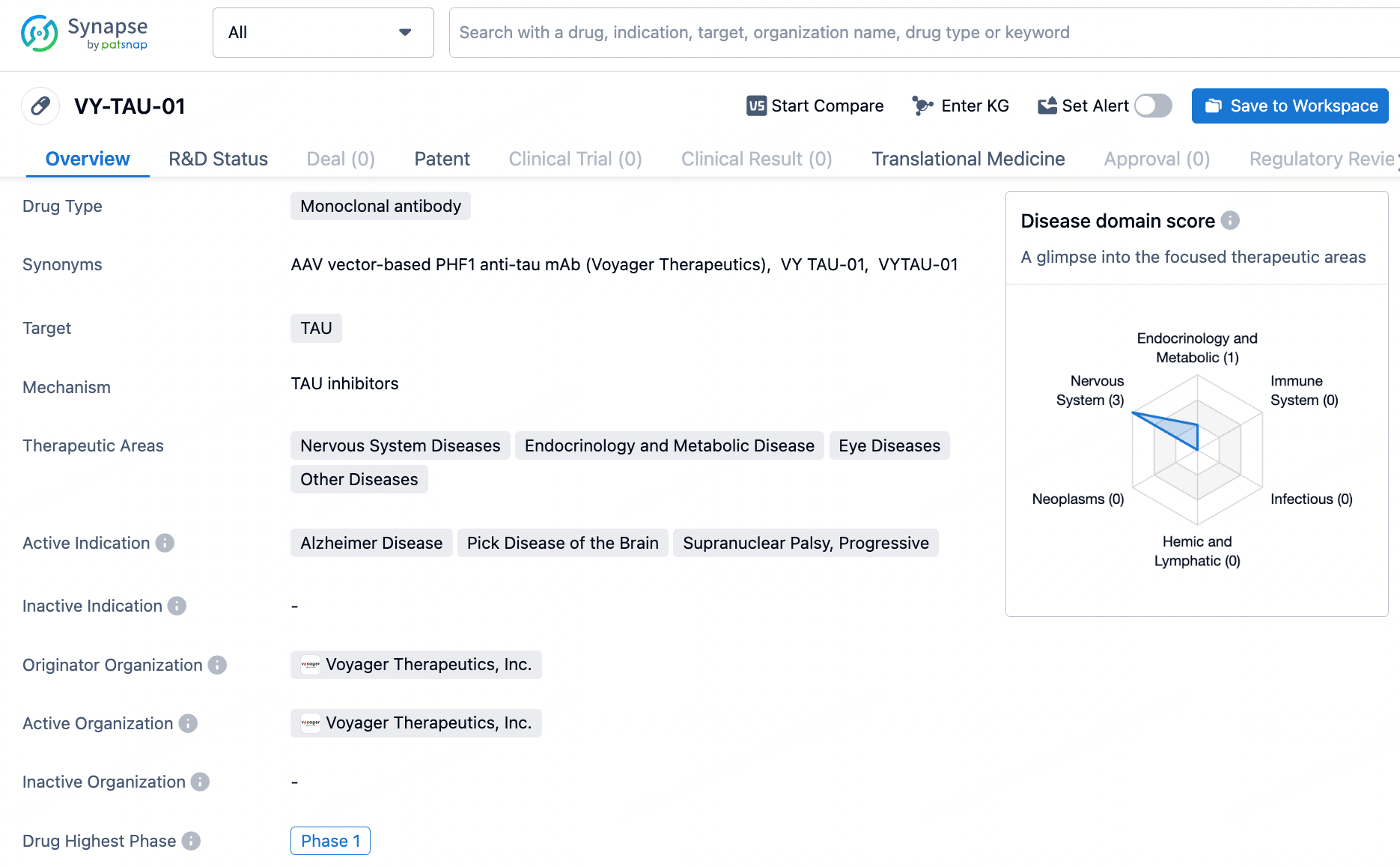
VY-TAU01 is an intravenously administered, humanized IgG4 monoclonal antibody designed to halt the spread of pathological tau, a factor closely linked to the progression and cognitive decline observed in Alzheimer's disease. Unlike earlier N-terminal targeting anti-tau antibodies that failed to show efficacy in clinical trials, VY-TAU01 focuses on a unique C-terminal epitope of tau and has shown strong inhibition of pathological tau spread in preclinical models. Further preclinical studies revealed that VY-TAU01 was well-tolerated and displayed a favorable pharmacokinetic profile following IV administration.
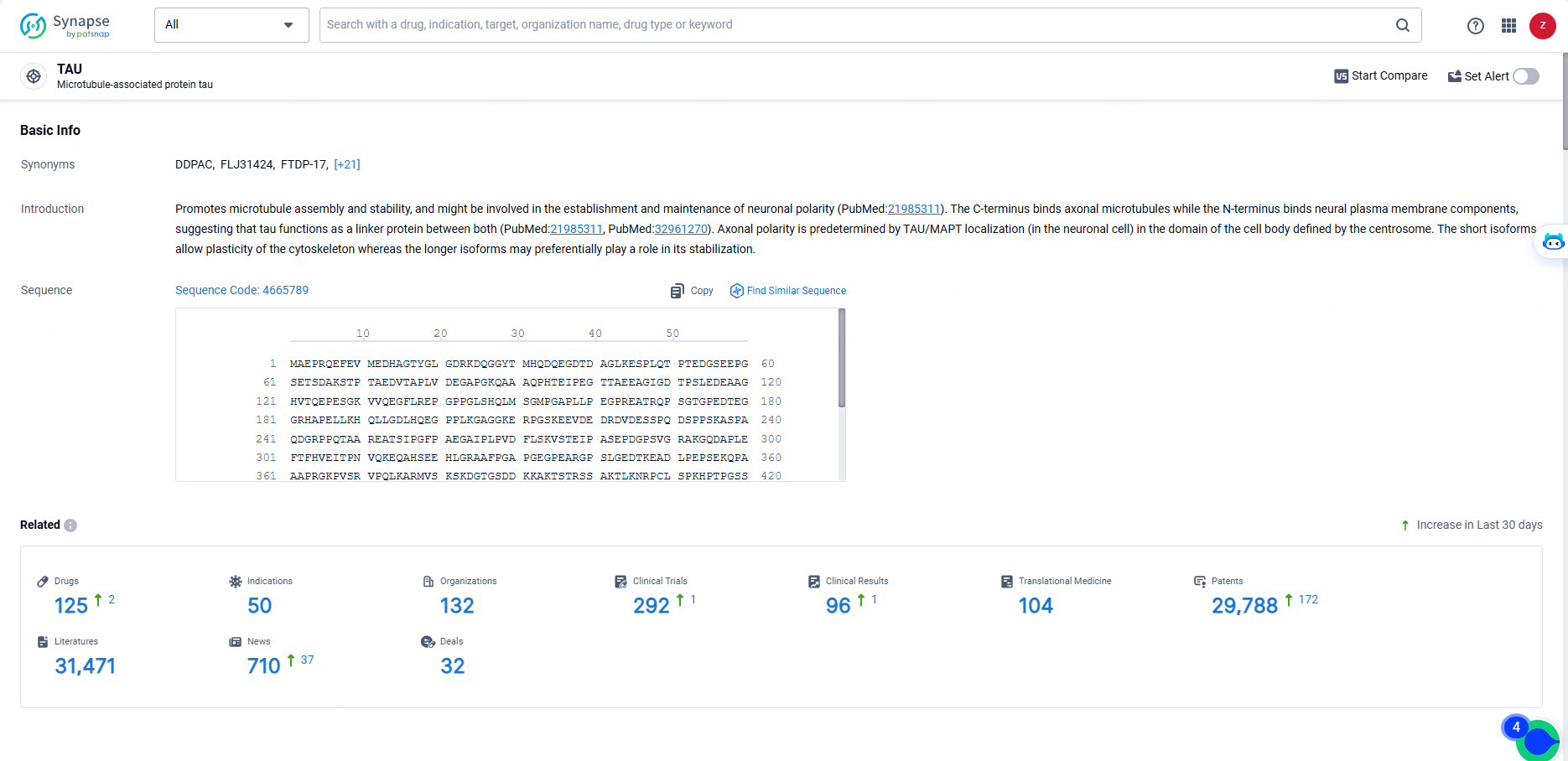
VY-TAU01, developed by Voyager Therapeutics, Inc., is a monoclonal antibody therapeutic targeting the TAU protein, which is implicated in several neurological conditions, metabolic and endocrinological diseases, ophthalmological disorders, and other ailments. Current indications for VY-TAU01 include Alzheimer’s Disease, Pick's Disease, Progressive Supranuclear Palsy, and related conditions. According to the most recent data, VY-TAU01 has entered Phase 1 clinical trials, signifying its initial evaluation in humans for safety and potential effectiveness.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 23, 2024, there are 125 investigational drugs for the TAU target, including 50 indications, 132 R&D institutions involved, with related clinical trials reaching 292, and as many as 29788 patents.
As VY-TAU-01 is in Phase 1 of clinical trials, it is still in the early stages of development. Further clinical trials will be necessary to assess the safety and efficacy of the drug in larger patient populations. If successful, VY-TAU-01 has the potential to address the unmet medical needs in the therapeutic areas of nervous system diseases, endocrinology and metabolic diseases, eye diseases, and other diseases.