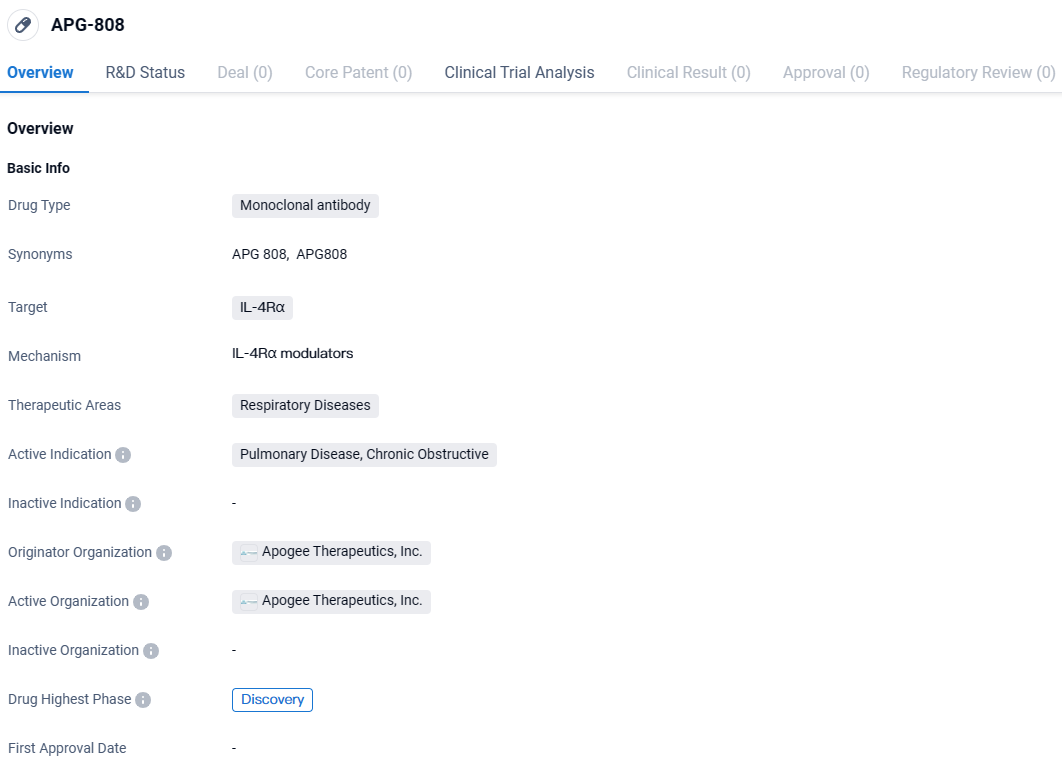
Apogee Therapeutics Begins Phase 1 Trial of APG808 for COPD and Inflammation
Apogee Therapeutics, Inc., has declared the commencement of administering the initial doses to healthy participants in its pioneering human study of APG808. This innovative therapy is a monoclonal antibody administered through subcutaneous injection and is designed with an enhanced half-life. APG808 specifically targets the IL-4Rα and is under development as a potential therapeutic option for individuals diagnosed with moderate-to-severe Chronic Obstructive Pulmonary Disease (COPD), asthma, and various other Inflammation & Immunology (I&I) conditions.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Following the release of encouraging early results from our Phase 1 trial of APG777, we are excited about the commencement of our Phase 1 APG808 study with volunteers in good health. This marks yet another stride forward in Apogee's commitment to creating a unique and improved lineup of biopharmaceuticals,” remarked Apogee's CEO, Dr. Michael Henderson.
Dr. Henderson added, “Accelerating the launch of our second exploratory health initiative, exceeding our original projections for the timeline, takes us further along in our mission to develop industry-leading biologics. These have the potential to transform treatment paradigms for patients contending with immune and inflammatory diseases while solidifying our reputation for reliable progress.”
The investigational molecule APG808 is a pioneering subcutaneous (SQ) agent with an extended half-life, directed against IL-4Rα. This target is known for its therapeutic relevance in up to eight distinct ailments related to Type 2 allergies. The binding affinity of APG808 for IL-4Rα is on par with the initial mAb DUPIXENT, shown through femtomolar level interactions. APG808's efficacy in impeding the IL-13/IL-4 signaling has been comparable to that of DUPIXENT across trio of in vitro assessments.
Chronic Obstructive Pulmonary Disease (COPD) remains an important health concern, progressing over time and currently affecting an estimated 10% of the worldwide demographic over 40. Even with modern treatment improvements, a significant number of individuals are still affected adversely by COPD.
Carl Dambkowski, M.D., Apogee’s Chief Medical Officer stated, “With APG777, we've already illustrated the advantages that come with refining the attributes of antibodies. We're optimistic about replicating this success with APG808. Drawing from comparative preclinical research, APG808 has shown efficacy closely matched to existing treatments but benefits from a substantially protracted half-life. This could herald a transformative change by allowing COPD patients potentially less frequent doses, thereby enhancing their overall well-being.”
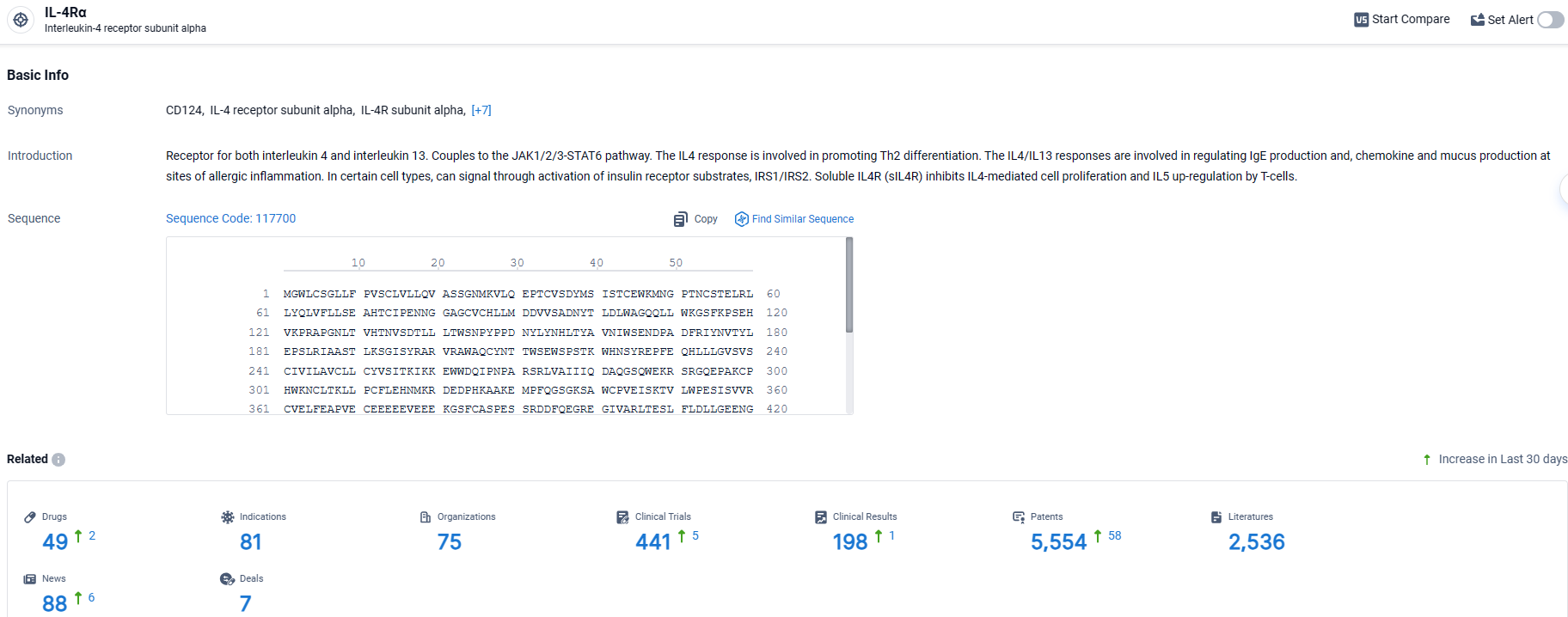
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 27, 2024, there are 49 investigational drugs for the IL-4Rα target, including 81 indications, 75 R&D institutions involved, with related clinical trials reaching 441, and as many as 2536 patents.
APG808 is a novel, subcutaneous extended half-life monoclonal targeting IL-4Rα, a target with clinical validation across eight Type 2 allergic diseases, for the potential treatment of chronic obstructive pulmonary disease, asthma and other inflammatory and immunology indications. Pending data from the Phase 1 trial, the company plans to initiate a potential Phase 1b trial in asthma with a data readout in in the first half of 2025 and a randomized, placebo-controlled Phase 2 clinical trial in patients with moderate-to-severe COPD in 2025.