Regeneron Announces Progress on Licensing Submission for Cancer Drug Odronextamab
Regeneron Pharmaceuticals, Inc. has declared the receipt of Complete Response Letters from the U.S. Food and Drug Administration concerning their submitted Biologics License Application for the drug odronextamab. These responses pertain to the application for the treatment of relapsed or refractory follicular lymphoma as well as for R/R diffuse large B-cell lymphoma, with both indications targeting patients who have undergone at least two prior lines of systemic therapy.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The primary concern regarding the authorization process pertains to the recruitment status for the confirmatory segments of the clinical studies. The Complete Response Letters (CRLs) – issued separately for Relapsed/Refractory Follicular Lymphoma (R/R FL) and Relapsed/Refractory Diffuse Large B-cell Lymphoma (R/R DLBCL) – raised no questions about the clinical effectiveness, safety profile, experimental design, proposed labeling, or production processes of odronextamab.
Regeneron is actively enrolling participants in a series of Phase 3 odronextamab trials under the ambit of the comprehensive OLYMPIA program targeting lymphoma. This program seeks to revolutionize the clinical approach for various subtypes of B-cell non-Hodgkin lymphoma, potentially advancing its use in initial treatments. The FDA, in endorsement of this initiative, mandated the inclusion of segments for both dose optimization and confirmatory evaluation.
While patient recruitment for the dose-optimization phase has commenced, the CRLs emphasize that the confirmatory phases need to be initiated, with clear agreements on the timelines for completion established before any plans to resubmit. Regeneron is dedicated to collaborating with the FDA and clinical researchers to expedite the availability of odronextamab for individuals suffering from R/R FL and R/R DLBCL. Future announcements about the progress of participant enrollment and the specifics of the regulatory schedule are expected later within the year.
The European Medicines Agency is currently conducting a review of odronextamab for the treatment of R/R DLBCL and R/R FL. Odronextamab has received the Orphan Drug Designation in the European Union for both DLBCL and FL, indicating its potential value in treating these rare diseases.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
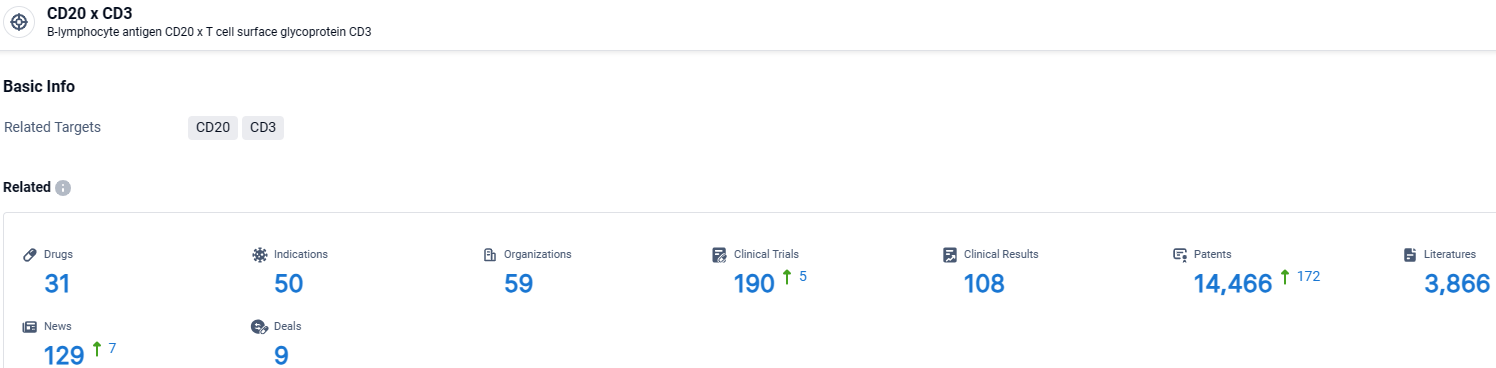
According to the data provided by the Synapse Database, As of March 27 2024, there are 31 investigational drugs for the CD20 and CD3 target, including 50 indications, 59 R&D institutions involved, with related clinical trials reaching 190, and as many as 14466 patents.
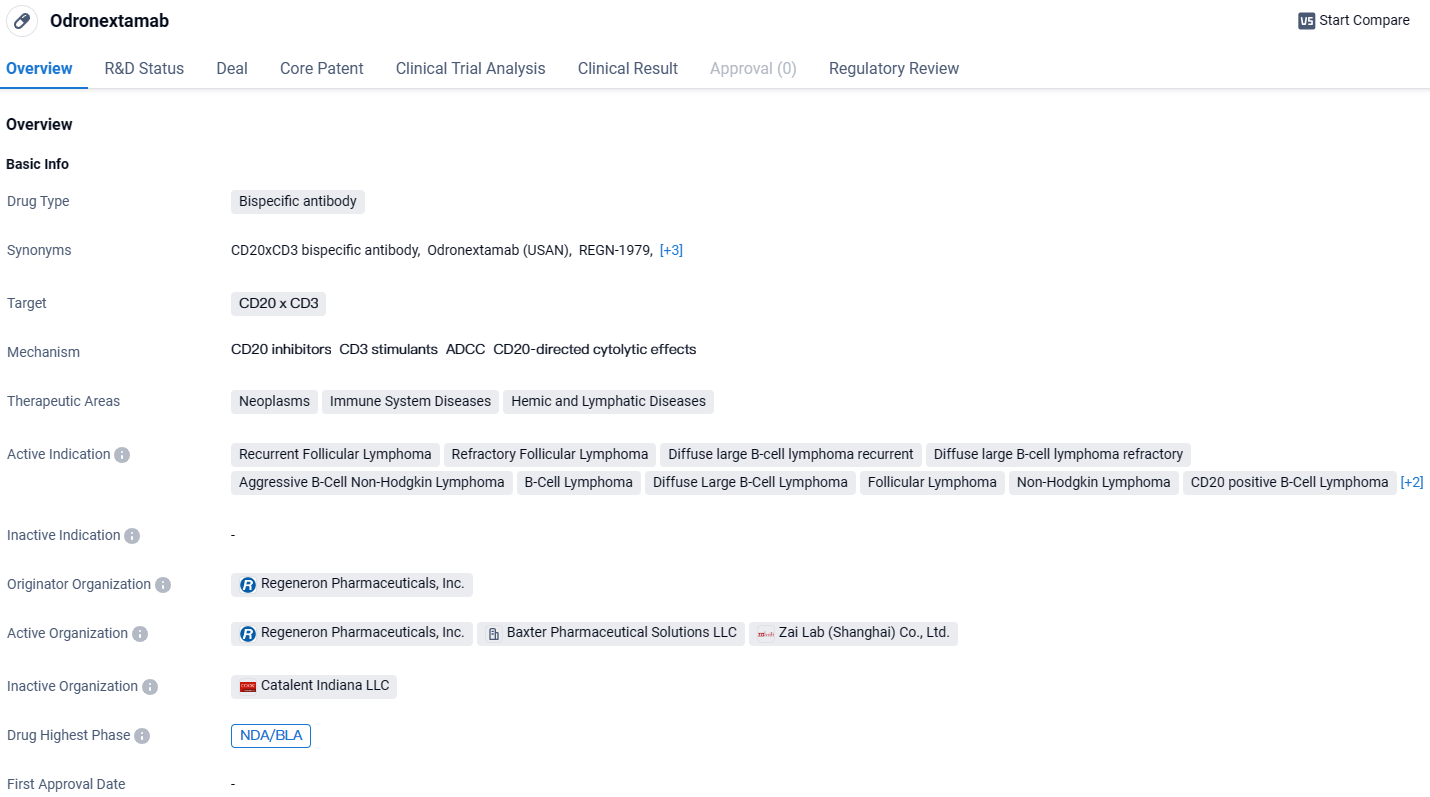
Odronextamab is a bispecific antibody drug developed by Regeneron Pharmaceuticals, Inc. It targets CD20 and CD3 and is indicated for various neoplastic, immune system, and hemic and lymphatic diseases. With its highest phase being NDA/BLA globally and Phase 3 in China, the drug is progressing towards potential approval and commercialization. The regulatory designations of priority review, fast track, and orphan drug further highlight its potential as a promising therapeutic option.