Argenx Announces FDA Approval for VYVGART Hytrulo in Treating CIDP
argenx SE, an international company focused on immunology, is dedicated to enhancing the quality of life for individuals afflicted with serious autoimmune disorders. The company recently revealed that the U.S. Food and Drug Administration has given approval for VYVGART Hytrulo (efgartigimod alfa and hyaluronidase-qvfc), intended for the treatment of adult patients diagnosed with chronic inflammatory demyelinating polyneuropathy.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
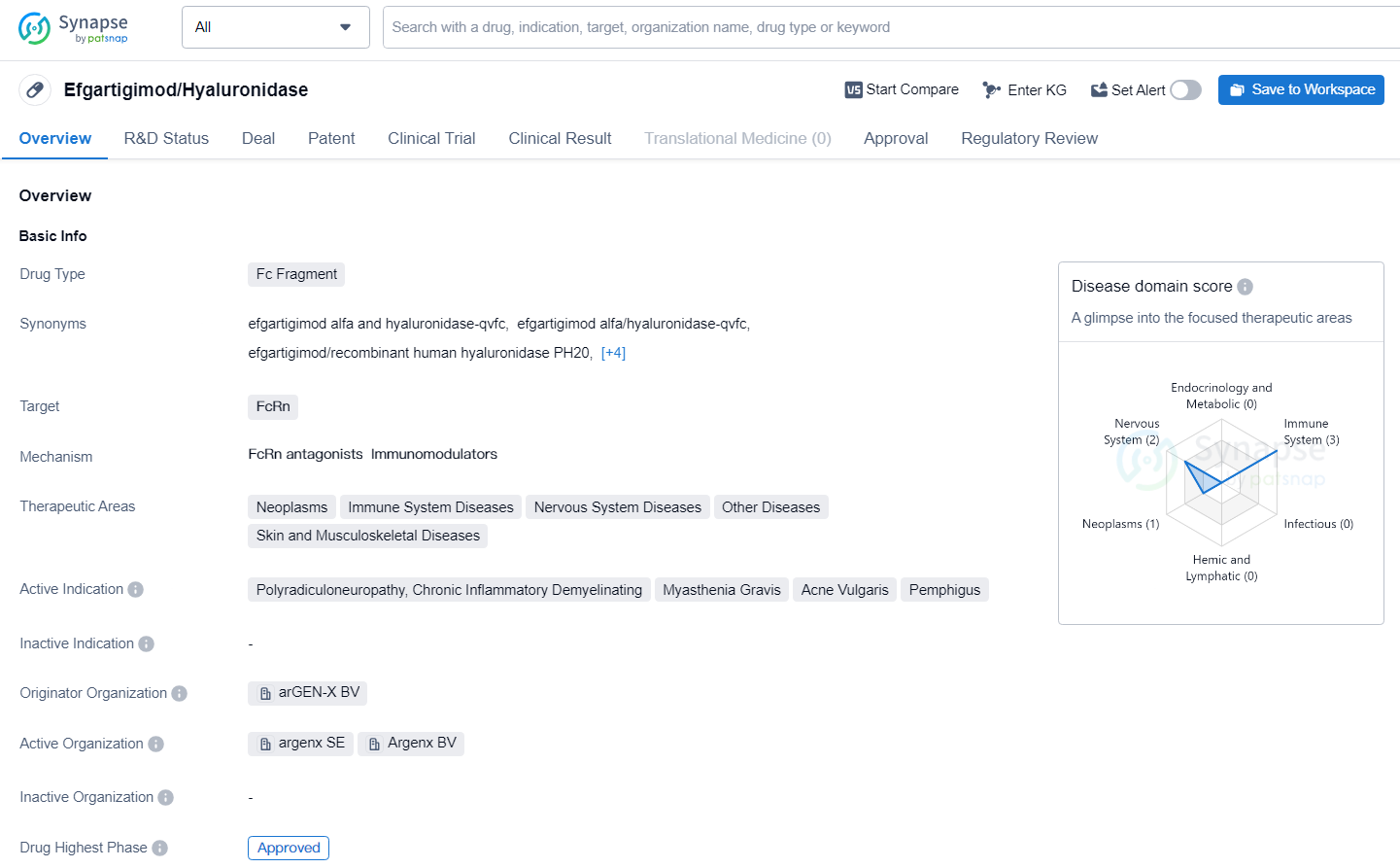
VYVGART Hytrulo has received approval for Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) as a weekly subcutaneous injection, administered over 30 to 90 seconds. This marks the first instance of an approved neonatal Fc receptor (FcRn) blocker specifically for CIDP treatment.
"At argenx, we are steadfast in our commitment to transforming scientific insights into patient-centric solutions for severe autoimmune diseases," stated Dr. Luc Truyen, Chief Medical Officer at argenx. "After decades of limited innovation, we are thrilled to introduce the first significant advance in CIDP treatment in over 30 years. VYVGART Hytrulo offers a precisely targeted approach that delivers notable benefits to patients. Today's FDA approval introduces a groundbreaking new therapy for CIDP patients, reinforcing the therapeutic promise of VYVGART Hytrulo and the potential of FcRn inhibition in managing IgG-mediated autoimmune conditions."
CIDP is a rare, debilitating, and often progressive autoimmune disorder impacting the peripheral nervous system. Patients grapple with significant mobility and sensory challenges, such as difficulty standing from a seated position, pain, fatigue, and frequent falls. Many eventually require wheelchairs and may be unable to work as the disease progresses. Approximately 85% of these patients need ongoing treatment, and nearly 88% of those treated experience lasting impairments and disabilities.
"CIDP patients contend with numerous daily challenges, and the fear of disease progression should not be one of them. This condition can severely impact quality of life, necessitating treatments that can be burdensome. The introduction of this new treatment option brings hope that patients can address their disease more effectively rather than merely managing symptoms. CIDP patients deserve diverse treatment options, and we are optimistic about a future with more choices for tailored and optimal care," said Lisa Butler, Executive Director of the GBS|CIDP Foundation.
The FDA's approval is derived from the ADHERE Study, the most extensive clinical trial conducted for CIDP to date. In this study, 69% of patients treated with VYVGART Hytrulo (with various prior treatments) exhibited clinical improvements in mobility, function, and strength. The ADHERE study successfully met its primary endpoint by showcasing a 61% reduction in relapse risk compared to placebo. Ninety-nine percent of the trial's participants opted for the open-label extension to continue receiving VYVGART Hytrulo. The safety results were consistent with the already established safety profile of VYVGART observed in previous clinical trials and real-world applications.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
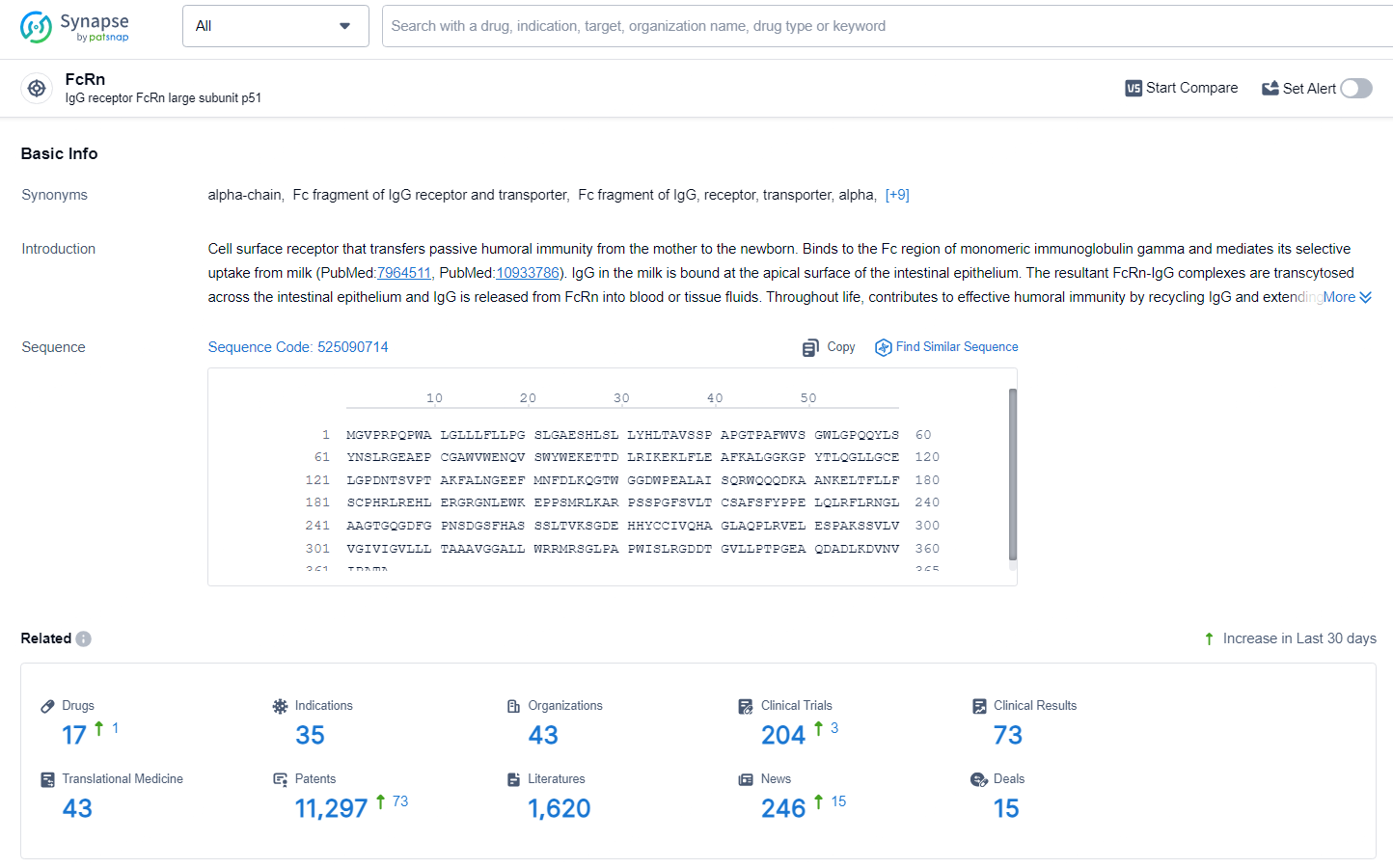
According to the data provided by the Synapse Database, As of June 27, 2024, there are 17 investigational drugs for the FcRn target, including 35 indications, 43 R&D institutions involved, with related clinical trials reaching 204, and as many as 11297 patents.
Efgartigimod/Hyaluronidase is a drug with a broad range of therapeutic areas and active indications, making it a potentially versatile treatment option. The drug is set to receive its first approval in the United States in June 2023 and is regulated under priority review as an orphan drug.