Takeda Gains European Commission Approval for FRUZAQLA in Treated Metastatic Colorectal Cancer
Takeda has revealed that the European Commission has given its approval for FRUZAQLA (fruquintinib) to be used as a monotherapy for adult patients suffering from metastatic colorectal cancer. This applies to individuals who have already undergone treatment with standard therapies commonly available, such as chemotherapies based on fluoropyrimidine, oxaliplatin, and irinotecan, as well as anti-VEGF and anti-EGFR agents. Moreover, these patients should have either shown progression or intolerance to treatments with trifluridine-tipiracil or regorafenib.
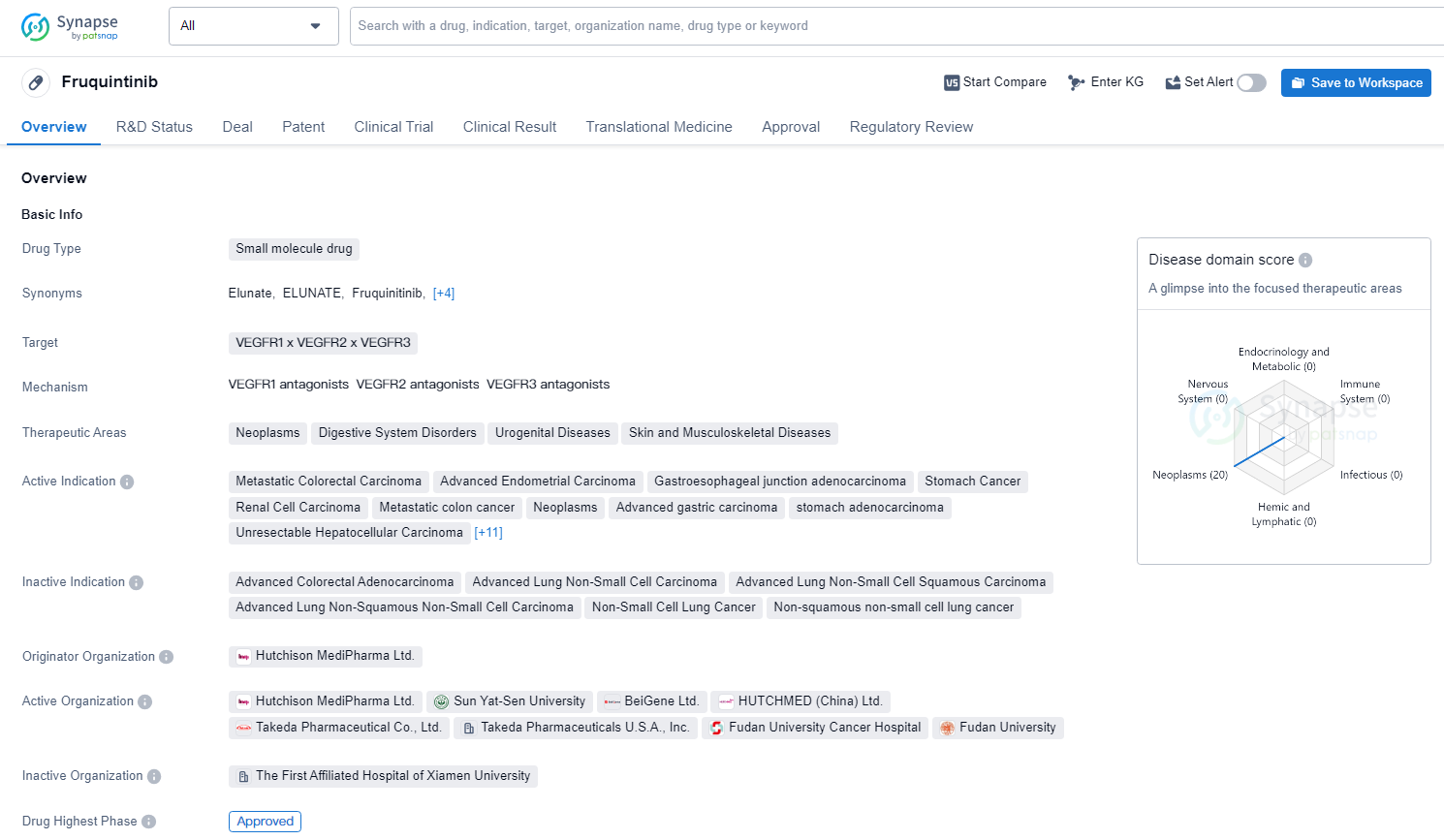
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The decision comes after an affirmative opinion from the Committee for Medicinal Products for Human Use on April 25, 2024, and subsequent endorsement by the U.S. Food and Drug Administration on November 8, 2023, for adults with mCRC previously treated with oxaliplatin- and irinotecan-based therapies.
"Individuals with metastatic colorectal cancer encounter numerous challenges, both due to the illness itself and the side effects of treatments. Considering the intricate nature of this disease, it is crucial to introduce innovative therapies like fruquintinib – an oral targeted agent that is chemotherapy-free. I eagerly anticipate this new option for suitable patients," stated Josep Tabernero, MD, PhD, director of Vall d´Hebron Institute of Oncology.
This approval is grounded on findings from the Phase 3 multi-regional FRESCO-2 trial. The study evaluated FRUZAQLA plus best supportive care against placebo plus BSC in patients with previously treated mCRC. FRESCO-2 successfully met all its primary and key secondary efficacy endpoints, demonstrating consistent benefits among patients treated with FRUZAQLA regardless of their prior therapies.
FRUZAQLA exhibited a manageable safety profile in the FRESCO-2 trial. Adverse reactions leading to treatment discontinuation occurred in 20% of patients receiving FRUZAQLA plus BSC, compared to 21% in those receiving placebo plus BSC. The data from FRESCO-2 was published in The Lancet in June 2023.
"This approval is a significant milestone for the colorectal cancer community in the EU. For the first time in over ten years, patients with previously treated metastatic colorectal cancer have a new targeted treatment option, which can be utilized irrespective of whether their tumors have actionable mutations," said Teresa Bitetti, president of the Global Oncology Business Unit at Takeda.
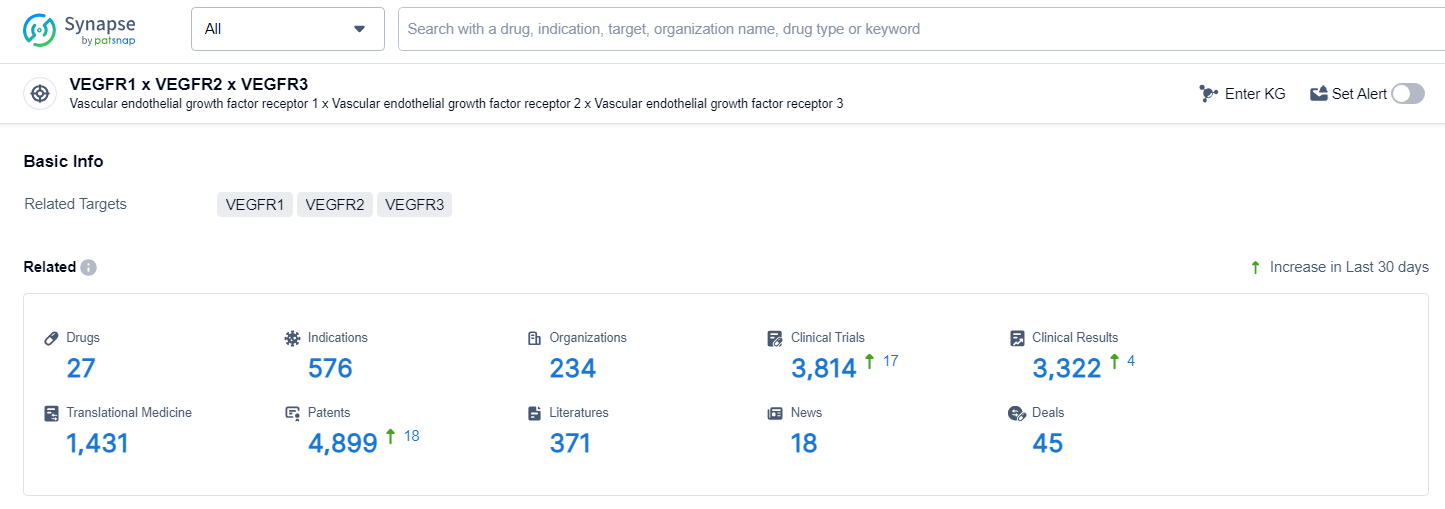
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 27, 2024, there are 27 investigational drugs for the VEGF target, including 576 indications, 234 R&D institutions involved, with related clinical trials reaching 3814, and as many as 4899 patents.
Fruquintinib is a small molecule drug that targets multiple VEGFRs and is used in the treatment of various cancers and other diseases. It has received approval in both the global market and China, with a first approval date in 2018. The drug has been granted priority review, fast track, and breakthrough therapy designations, indicating its potential for significant therapeutic advancement in the field of biomedicine.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!