Arialys Therapeutics Begins Phase 1 Trial of ART5803 for Autoimmune Neuropsychiatric Disorders
Arialys Therapeutics, a biotechnology firm at the clinical stage, is at the forefront of developing innovative precision treatments for autoimmune neuropsychiatric conditions. The company recently announced the commencement of its first clinical trial for ART5803, which involves administering doses to healthy participants. ART5803 is a candidate monoclonal antibody specifically aimed at counteracting pathogenic autoantibodies that attack the NMDA receptor (NMDAR). The presence of these harmful anti-NMDAR autoantibodies contributes to anti-NMDAR encephalitis (ANRE), a severe and inadequately addressed rare illness that currently lacks any authorized treatment options. Additionally, recent studies have suggested a connection between anti-NMDAR autoantibodies and other neuropsychiatric disorders, including schizophrenia and dementia. Arialys plans to launch a Phase 2a proof of concept clinical study for ART5803 targeting ANRE in the latter half of 2025.
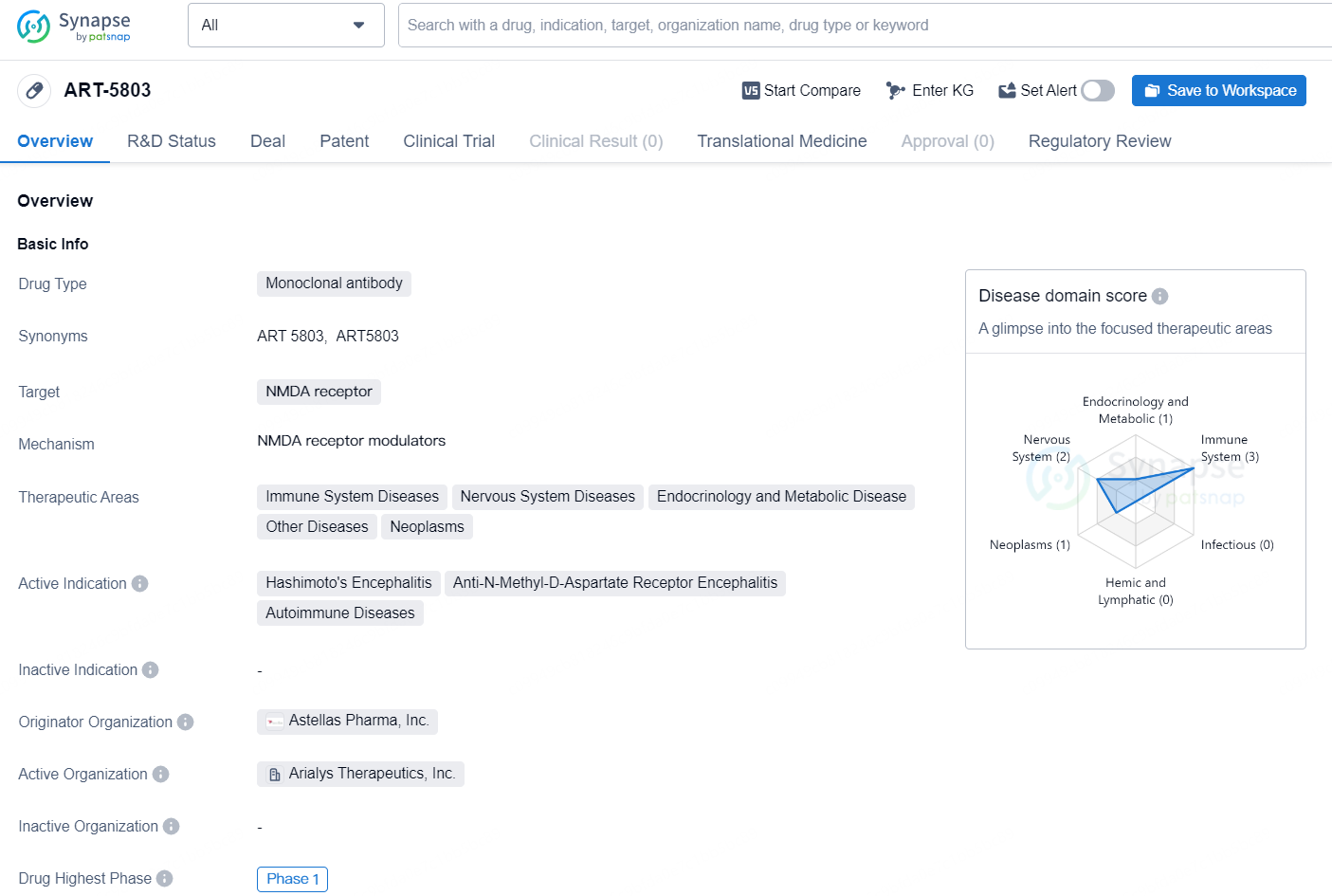
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“ART5803 represents a groundbreaking precision therapeutic option, and launching its clinical development marks a significant and thrilling advancement toward effectively addressing anti-NMDAR autoimmune neuropsychiatric disorders,” stated Peter Flynn, Ph.D., the President and CEO of Arialys Therapeutics. “Simultaneously with our clinical initiatives focused on ANRE, we are assessing autoantibody levels and exploring the therapeutic efficacy of ART5803 in individuals experiencing a wider range of neuropsychiatric disorders, including schizophrenia and dementia.”
The Phase 1 clinical trial of ART5803 is structured as a double-blind, placebo-controlled study involving first-time human participants receiving single-ascending doses. This trial will take place with the cooperation of Nucleus Network in Melbourne, Australia, and is registered under ClinicalTrials.gov Identifier: NCT06575153. The main objectives of the study are to assess the safety, tolerability, and pharmacokinetics (PK) of ART5803, with an anticipated enrollment of around 40 subjects.
If the results from the safety, tolerability, and PK evaluations in healthy volunteers are positive, Arialys plans to launch a Phase 2a proof-of-concept clinical trial for ART5803 in late 2025, targeting patients diagnosed with ANRE initially.
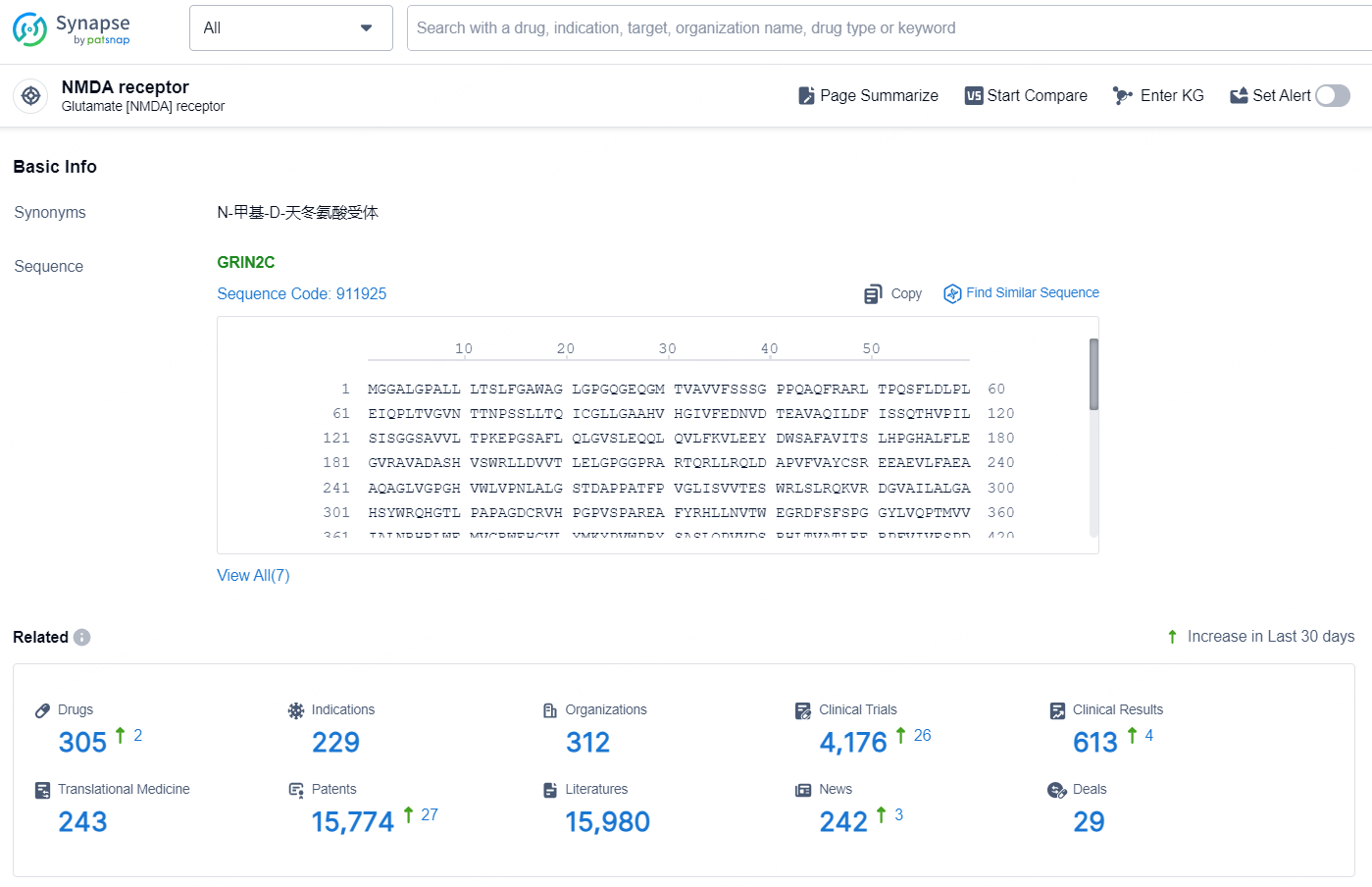
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 10, 2024, there are 305 investigational drug for the NMDA target, including 229 indications, 312 R&D institutions involved, with related clinical trials reaching 4176, and as many as 15774 patents.
ART-5803 is a monoclonal antibody drug developed by Astellas Pharma, Inc. The drug targets the NMDA receptor and is indicated for the treatment of a range of diseases related to the immune system, nervous system, endocrinology and metabolism, as well as neoplasms. Specifically, it is active in the treatment of Hashimoto's Encephalitis, Anti-N-Methyl-D-Aspartate Receptor Encephalitis, and other autoimmune diseases.