Arrowhead Advances RNAi Therapy Plozasiran to Phase 3 CAPITAN Trial in Cardiovascular Study
Arrowhead Pharmaceuticals, Inc. revealed their intention to proceed with the development of investigational plozasiran, moving it into a Phase 3 cardiovascular outcomes trial named CAPITAN. This decision follows favorable outcomes recently disclosed from several clinical studies across three different patient groups, including the crucial Phase 3 PALISADE trial involving individuals genetically confirmed or clinically diagnosed with familial chylomicronemia syndrome.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The Phase 2 SHASTA-2 trial, targeting individuals with severe hypertriglyceridemia, was documented in JAMA Cardiology. Similarly, the Phase 2 MUIR trial, focusing on patients with mixed hyperlipidemia, was reported in the New England Journal of Medicine.
"Our second cardiometabolic agent, zodasiran, has shown highly promising outcomes, including reductions in triglyceride levels and significant, sustained decreases in triglyceride-rich lipoproteins, remnants, and total atherogenic lipoproteins. These results encourage further investigation in Phase 3 trials. However, after evaluating both candidates and considering resource distribution, we've decided to independently advance plozasiran. At this time, further development of zodasiran will only proceed if we find a compatible partner for development and commercialization," Dr. Given concluded.
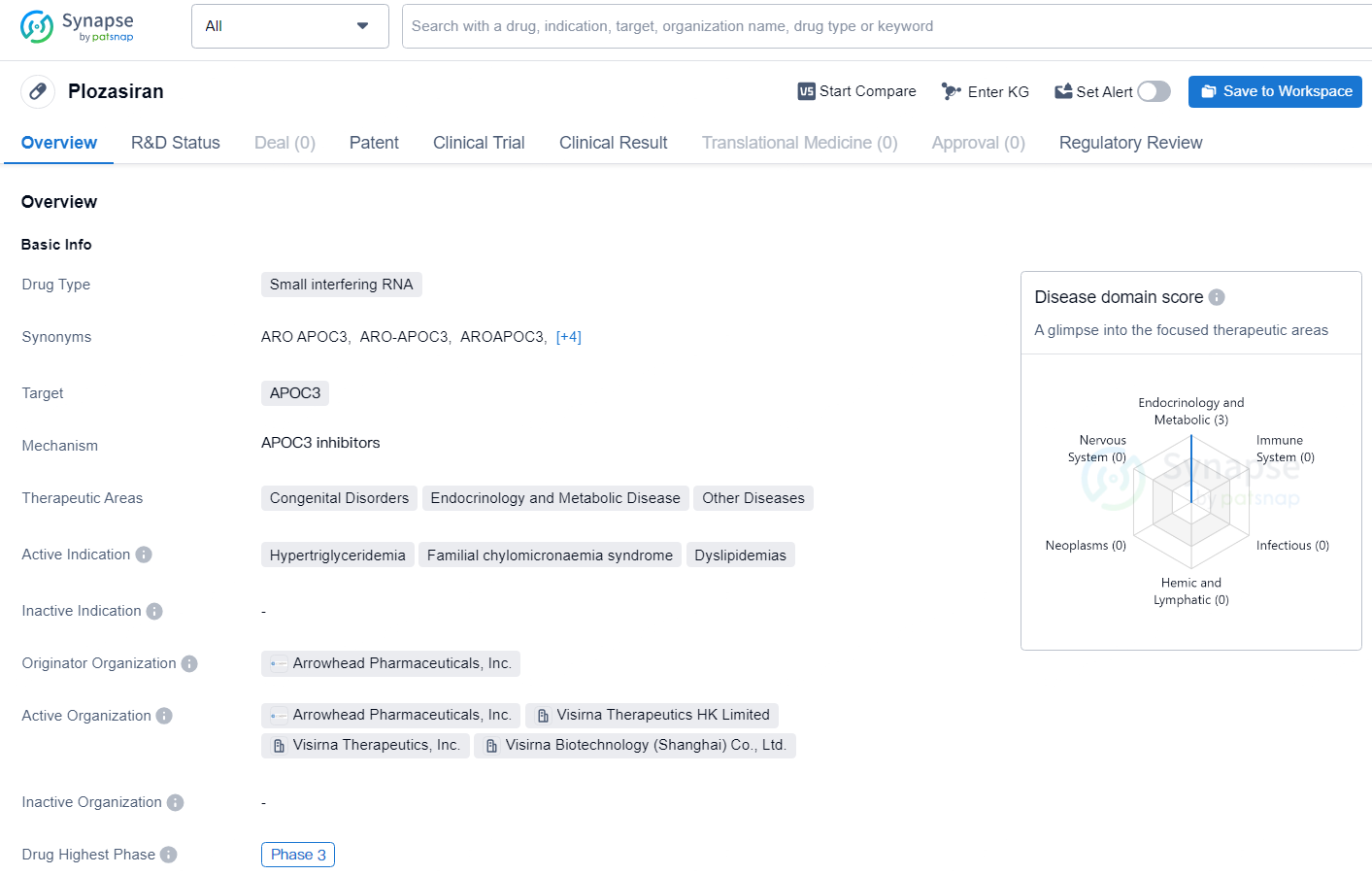
Plozasiran, formerly known as ARO-APOC3, is a pioneering investigational RNA interference therapy designed to lower the production of Apolipoprotein C-III (APOC3), a component of triglyceride-rich lipoproteins and a key player in triglyceride metabolism.
APOC3 elevates triglyceride levels in the blood by impeding the breakdown of TRLs by lipoprotein lipase and the hepatic receptor-mediated uptake of TRL remnants. The primary aim of plozasiran treatment is to decrease APOC3 levels, which in turn reduces triglycerides and normalizes lipid levels.
In various clinical trials, investigational plozasiran has shown reductions in triglycerides and multiple atherogenic lipoproteins in patients with familial chylomicronemia syndrome, severe hypertriglyceridemia, and mixed hyperlipidemia.
To date, plozasiran has exhibited a favorable safety profile, with treatment-emergent adverse events reflecting the comorbidities and underlying conditions of the study populations. Currently, plozasiran is being evaluated in the PALISADE Phase 3 clinical trial involving patients with FCS, which has recently concluded, the ongoing SHASTA-3,4,5 Phase 3 trials in patients with SHTG, and the forthcoming CAPITAN Phase 3 trial assessing patients with mixed hyperlipidemia.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
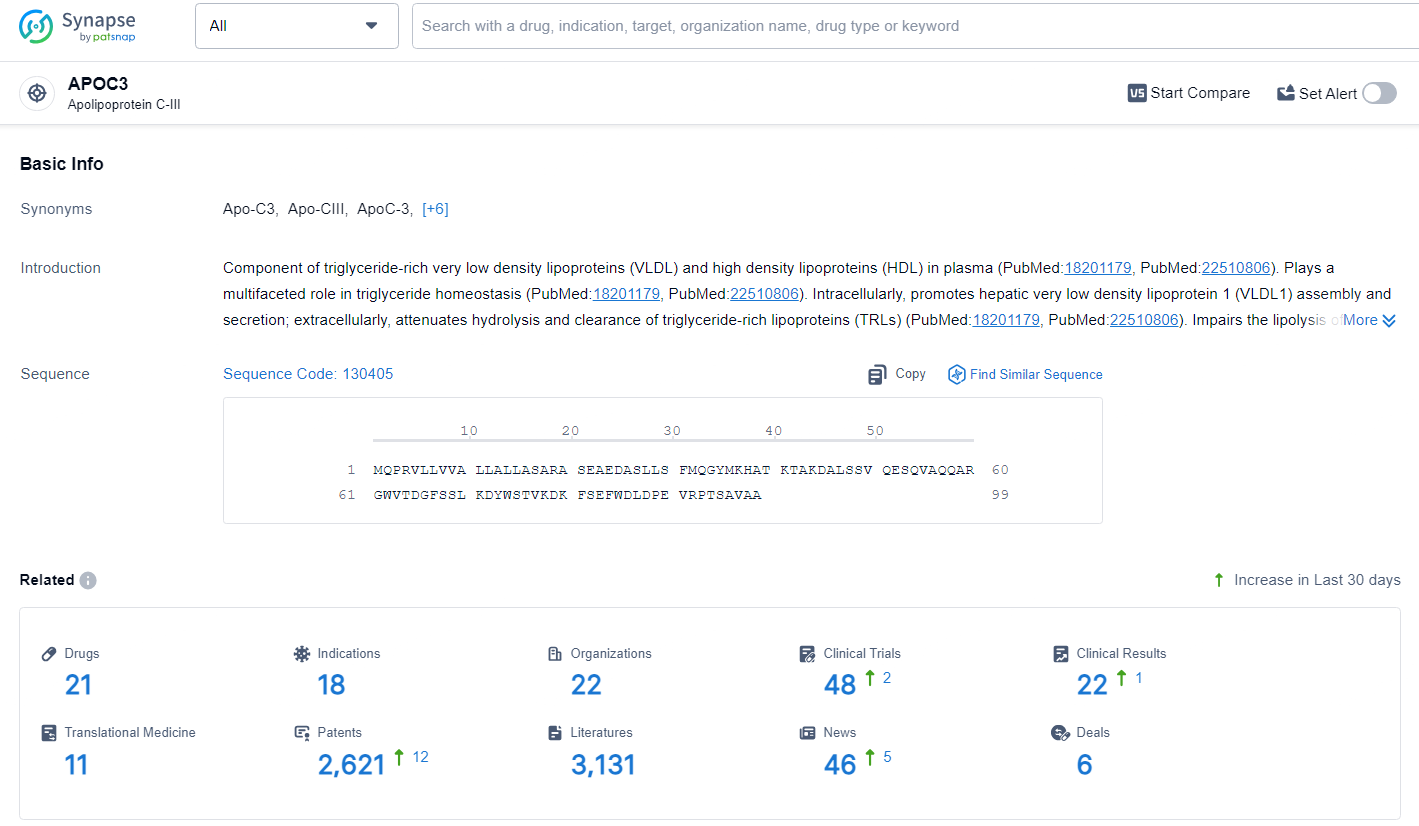
According to the data provided by the Synapse Database, As of July 1, 2024, there are 21 investigational drugs for the APOC3 target, including 18 indications, 22 R&D institutions involved, with related clinical trials reaching 48, and as many as 2621 patents.
Plozasiran is a promising siRNA drug targeting APOC3, currently in Phase 3 clinical trials for the treatment of hypertriglyceridemia and related conditions. With regulatory designations such as Fast Track, Orphan Drug, and Breakthrough Therapy, Plozasiran has the potential to address unmet medical needs and make a significant impact in the field of lipid metabolism disorders.