Artax Begins Phase 2a Psoriasis Trial with First Participant Dosed with AX-158
Artax Biopharma, Inc., a biotech enterprise in the clinical phase dedicated to revolutionizing therapies for autoimmune conditions, has declared the commencement of dosing in their Phase 2a study. This trial is designed to assess the tolerability and biomarker reactions to AX-158 as a pioneering test of the drug's mechanism in treating psoriasis. Artax Biopharma anticipates the availability of trial findings later in the latter part of the current year.
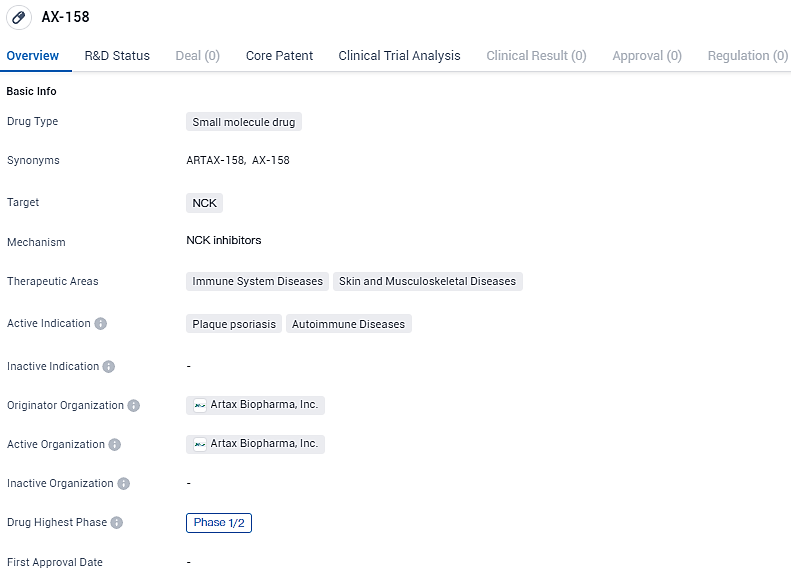
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The anticipation for the experimental drug AX-158 is high, given that it could be the inaugural agent in the Nck inhibitor category. "The preliminary laboratory findings give us confidence that AX-158 might lead to positive effects in treating autoimmune disorders, sidestepping the typical adverse reactions and immune dampening seen with current treatments," commented Artax's CEO, Dr. Rob Armstrong. "We are keenly awaiting the results from the ongoing psoriasis Phase 2a study, which we'll learn about by the year's end."
Immunomodulators like AX-158 assist in providing a balanced regulation of the immune system, addressing the primary mechanisms that fuel autoimmune pathologies. As a novel entity among Nck inhibitors, AX-158 may redefine the therapeutic approach in managing autoimmune conditions. It targets the Nck pathway, crucial for the function of the immune system, and realigns how the body's T-cell receptors operate.
This realignment ensures that the immune system remains active only against genuine threats of disease, thereby averting unwarranted self-targeting and avoiding the reduction of immune defenses that typically leaves individuals prone to infections.
James G. Krueger, M.D., Ph.D., the director of the Laboratory for Investigative Dermatology at The Rockefeller University and a member of the Artax Scientific Advisory Board, remarked, "The pioneering category of Nck inhibitors, exemplified by AX-158, holds the promise of revolutionizing autoimmune disease management. It does so by fine-tuning the TCR mechanisms so that the immune reaction is reserved for true pathogenic challenges." Dr. Krueger continued, "It's heartening to anticipate the potential shift from merely curbing immune responses to a more targeted intervention. I look forward to witnessing the impact this novel therapeutic approach has on clinical biomarkers and the improvement in individuals' health."
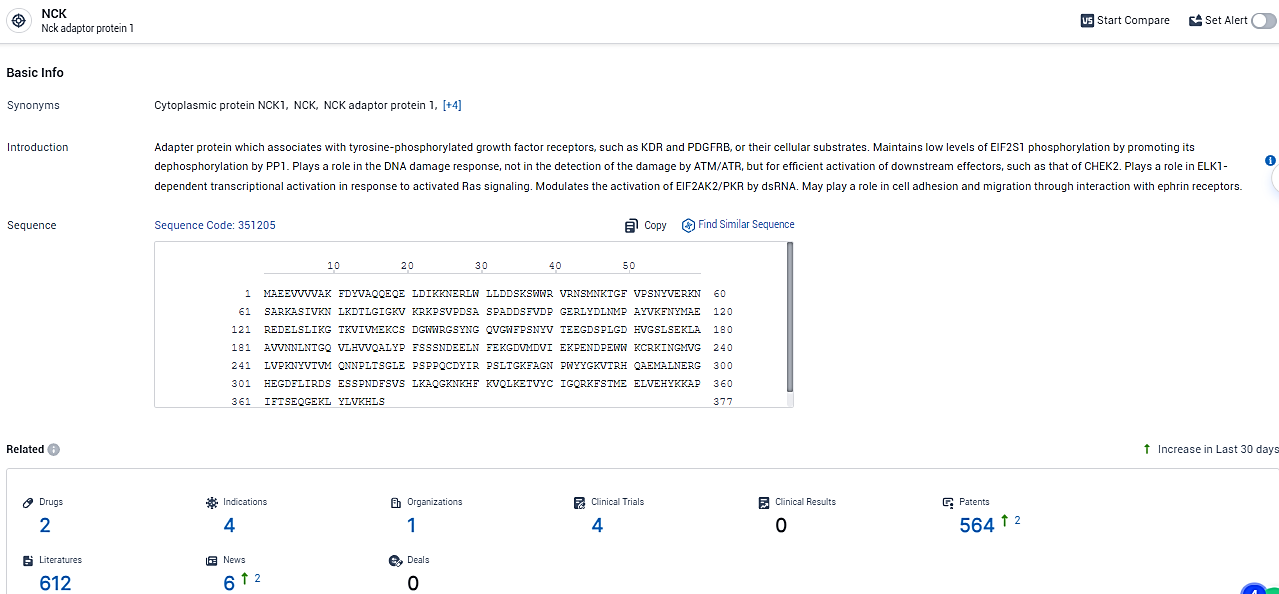
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 20, 2024, there are 2 investigational drugs for the NCK target, including 4 indications, 1 R&D institutions involved, with related clinical trials reaching 4, and as many as 564 patents.
AX-158 targets NCK. It is currently in Phase 1/2 of clinical development and is being investigated for its potential use in treating immune system diseases, specifically plaque psoriasis and autoimmune diseases. Further research and clinical trials are needed to determine the drug's efficacy and safety profile.