Otsuka Announces AVP-786 Trial Results for Alzheimer's Agitation
Otsuka Pharmaceutical Development & Commercialization, Inc., along with its holding entity, Otsuka Pharmaceutical Co., Ltd., have released preliminary findings from the third phase of clinical research for AVP-786. This medication is being evaluated for its effectiveness in managing symptoms of restlessness and unease commonly seen in patients suffering from Alzheimer’s-related dementia.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
No substantial difference was observed in the primary measurement of effectiveness, which is the average alteration from the initial baseline to the 12-week mark using the Cohen-Mansfield Agitation Inventory overall score when comparing AVP-786 with a placebo.
The comprehensive outcomes of the trial have yet to be disclosed. Detailed predefined and additional exploratory evaluations of the collected data will be undertaken to fully ascertain AVP-786's capabilities in addressing agitated behaviors in patients experiencing dementia of the Alzheimer’s type. Otsuka is planning to release these clinical trial findings for expert review and publication at a subsequent time.
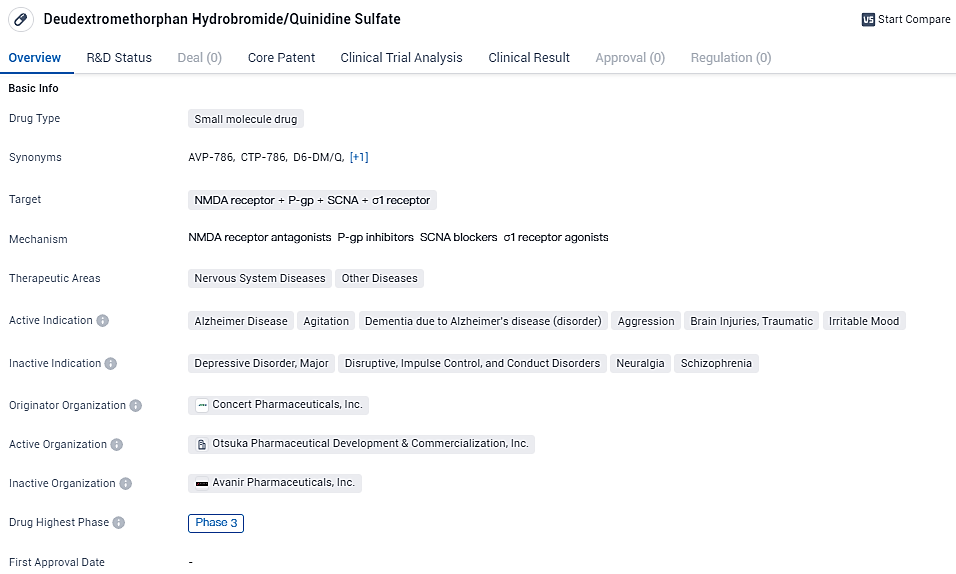
"In the year 2023, Otsuka distinguished itself as the pioneer in securing approval for a therapeutic for this specific demographic, and we maintain our commitment to enhancing and pioneering within this realm," stated John Kraus, M.D., Ph.D., who serves as the executive vice president and chief medical officer at Otsuka. "We extend our heartfelt appreciation to all the individuals who participated in the study, their families, and the clinical professionals who engaged in the investigation and contributed to this scientific endeavor."
The neuropsychiatric complication of agitation commonly arises in about half the individuals diagnosed with Alzheimer’s form of dementia. This condition significantly detracts from the life quality of the affected individuals, their relatives, and support personnel. Agitation in patients with Alzheimer’s type dementia encompasses a spectrum of behavioral manifestations, including repetitive motion, non-verbal communications, use of strong language, vocal outbursts, pushing, and physical confrontations. The presence of these behavioral indicators also reliably forecasts the necessity for care in a nursing home setting.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
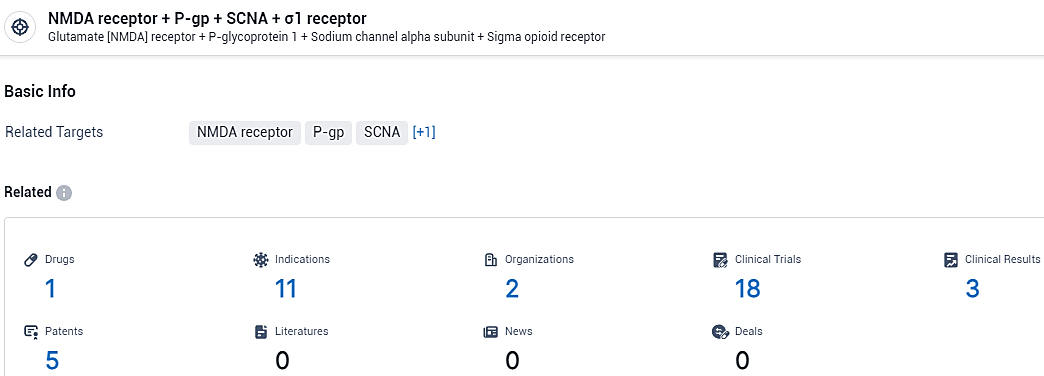
According to the data provided by the Synapse Database, As of February 20, 2024, there are 1 investigational drugs for the NMDA receptor + P-gp + SCNA + σ1 receptor target, including 11 indications, 2 R&D institutions involved, with related clinical trials reaching 18, and as many as 5 patents.
AVP-786 is a combination of deudextromethorphan hydrobromide and quinidine sulfate, a CYP2D6 inhibitor. Deuteration was observed to significantly reduce susceptibility to cytochrome P450 enzyme metabolism thereby increasing the bioavailability. The drug is currently in Phase 3 of clinical development, indicating its advanced stage of testing and potential for future therapeutic use.