ARTBIO collaborates with PharmaLogic to distribute Lead-212 infused drug AB001 across NY and neighboring regions
PharmaLogic Holdings Corp., a prominent enterprise specializing in the contract development and production of radiopharmaceuticals, has reached an agreement with ARTBIO, an innovative company in the clinical phase that is advancing novel alpha-emitting radioligand treatments. The deal pertains to the production and provision of ARTBIO's radiopharmaceutical product in development, AB001, which utilizes lead-212 and is targeted at addressing prostate cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In accordance with the signed partnership arrangement, PharmaLogic has committed to supporting ARTBIO by contributing radiochemistry expertise and providing the completed radiopharmaceutical product of AB001 for the upcoming initial and second-phase clinical evaluations, utilizing the specialized AlphaDirect™ 212Pb separation technique from ARTBIO, which will be facilitated at PharmaLogic's New York site.
Emanuele Ostuni, Ph.D., CEO of ARTBIO, remarked on the collaboration by saying, "Joining forces with PharmaLogic enhances our initiatives significantly; their extensive skill set in the production of radiopharmaceuticals and diagnostic tools dovetails with our innovative AlphaDirect™ method for 212Pb isolation and our pursuit of pioneering alpha radioligand treatment modalities. The union of our expertise and our mutual dedication to advancing patient care reinforces our capacity to bring pioneering alpha radioligand therapies directly to those in need."
A notable alpha-emitting isotope in the medical field, 212Pb has drawn interest for its potential role in precision medicine, specifically in the area of targeted alpha radioligand therapy, thanks to its favorable half-life and other unique characteristics. Emerging research on 212Pb-labeled radiopharmaceuticals shows considerable potential, suggesting that 212Pb could provide solutions to persistent medical challenges.
D. Scott Holbrook, Chief Strategy Officer and General Manager of PharmaLogic, expressed enthusiasm about the collaboration with ARTBIO for producing and delivering AB001 for the upcoming clinical evaluations, stating, "Aligned with our core objective to transition groundbreaking radiopharmaceuticals to market for patient benefit, this partnership with ARTBIO is a stride towards enhancing our impact within the therapeutic radiopharmaceutical space, with the ultimate aim of elevating patient care standards."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
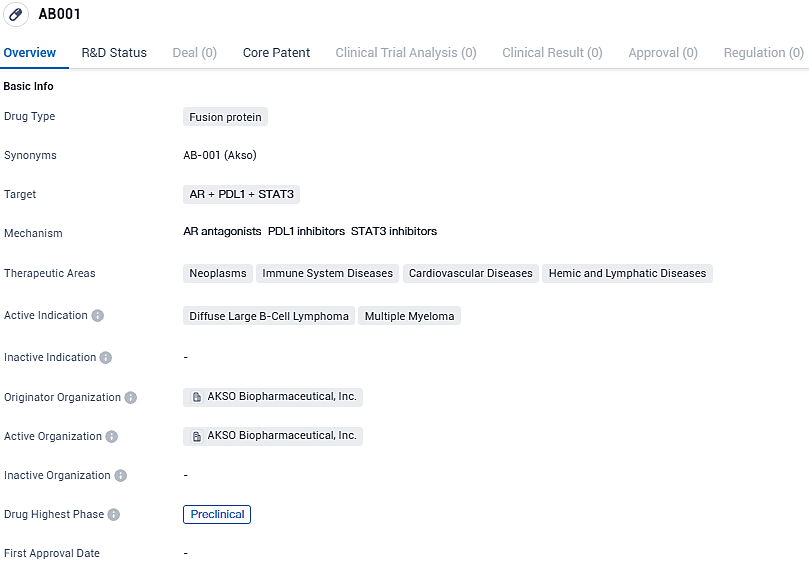
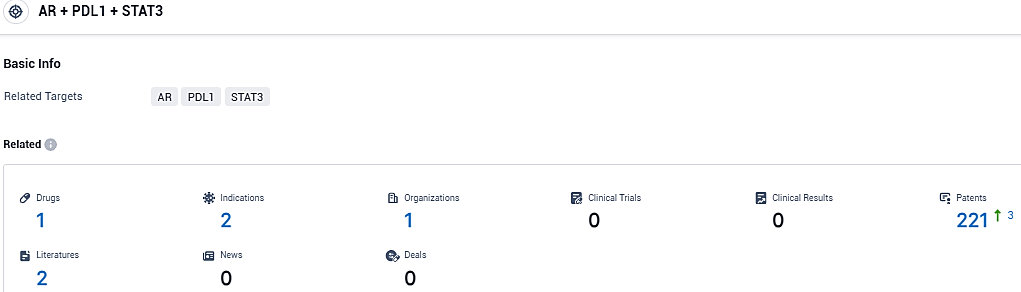
According to the data provided by the Synapse Database, As of January 18, 2024, there are 1 investigational drugs for the AR and PDL1 and STAT3 target, including 2 indications, 1 R&D institutions involved, and as many as 221 patents.
AB001 targets AR, PDL1, and STAT3 and aims to treat neoplasms, immune system diseases, cardiovascular diseases, and hemic and lymphatic diseases. The drug is indicated for DLBCL and multiple myeloma and is currently in the preclinical phase of development.