FDA Approves Merck's KEYTRUDA with Chemoradiotherapy for Stage III-IVA Cervical Cancer Based on FIGO 2014 Criteria
The pharmaceutical giant Merck, which operates under the brand name MSD in regions beyond the United States and Canada, has declared that the American Food and Drug Administration granted authorization for the use of KEYTRUDA. This treatment, which inhibits the PD-1 pathway, is now approved to be used in tandem with chemoradiotherapy to manage cases of cervical cancer ranging from Stage III to IVA, as classified by FIGO's 2014 standards.
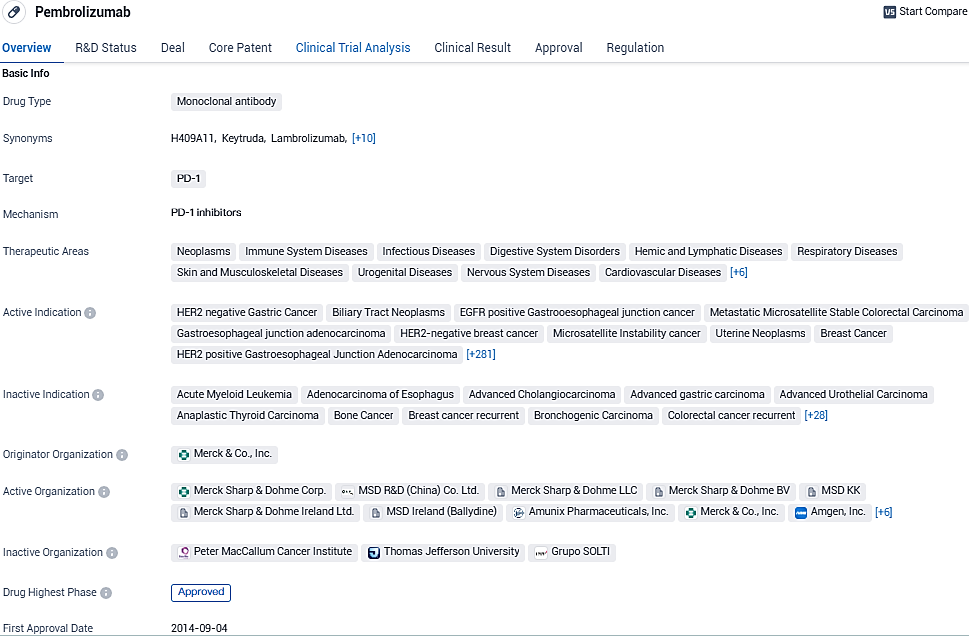
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The recent authorization is anchored in findings from the KEYNOTE-A18 Phase III study. Here, the combination of KEYTRUDA and CRT yielded an enhanced rate of progression-free survival, slashing the likelihood of disease worsening or mortality by 41% in contrast to the CRT plus placebo group. This was observed in subjects with FIGO 2014 Stage III-IVA cervical cancer. The median PFS had not been established for any cohort at data analysis. We now celebrate the third cervical cancer indication for KEYTRUDA and its 39th approval in the United States.
"Introducing KEYTRUDA in conjunction with chemoradiotherapy signifies a pivotal advancement, offering patients contending with FIGO 2014 Stage III-IVA cervical cancer an unprecedented anti-PD-1 therapy choice," remarked Dr. Bradley Monk, a renowned oncologist and academic in obstetrics and gynecology with affiliations to the University of Arizona's College of Medicine and Creighton University School of Medicine.
Treatment with KEYTRUDA poses risks like immune-mediated adverse effects, which may be grave or even lethal. These complications can arise in any system or tissue and could impact multiple systems at once. They have the potential to materialize any time during or following KEYTRUDA therapy and range from pneumonitis and colitis to hepatitis, various endocrinopathies, nephritis, skin reactions, rejection of transplanted solid organs, and issues post-allogeneic hematopoietic stem cell transplant.
This document does not exhaustively list all severe and potentially fatal immune-mediated reactions. To mitigate risks associated with KEYTRUDA, prompt detection and management of these immune-mediated effects are critical. Depending on the reaction severity, treatment with KEYTRUDA might need to be interrupted or stopped entirely, and the administration of corticosteroids may be considered as deemed necessary.
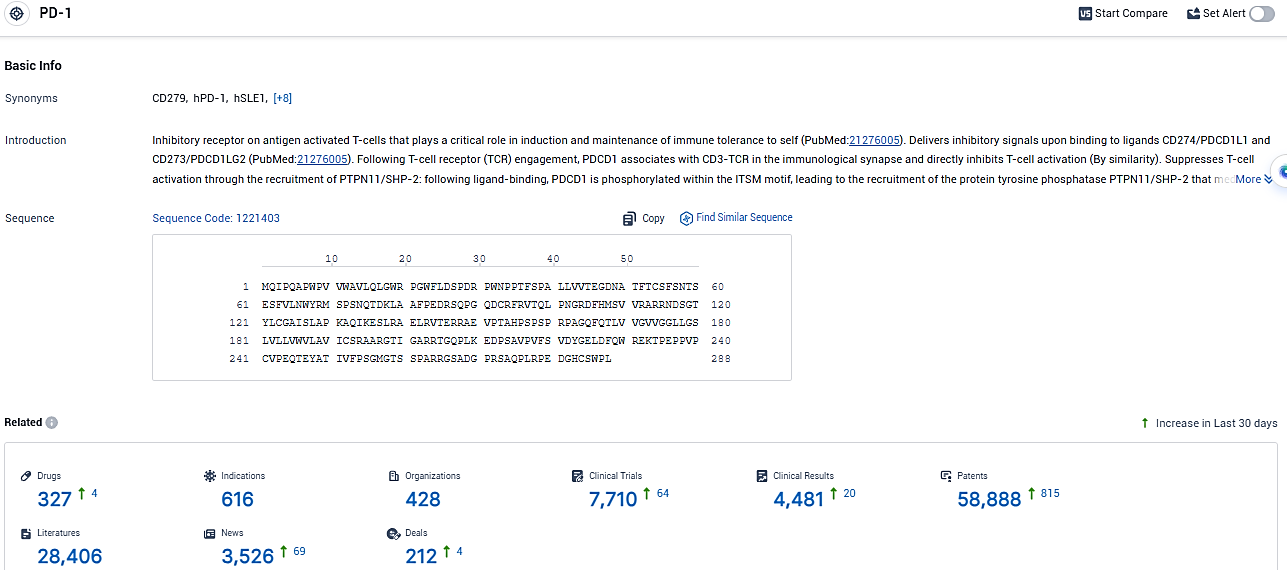
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 18, 2024, there are 327 investigational drugs for the PD-1 target, including 616 indications, 428 R&D institutions involved, with related clinical trials reaching 7710, and as many as 58888 patents.
KEYTRUDA is an anti-programmed death receptor-1 therapy that works by increasing the ability of the body’s immune system to help detect and fight tumor cells. In the U.S., KEYTRUDA has two additional approved indications in cervical cancer: in combination with chemotherapy. Its approval in multiple therapeutic areas and its designation as a breakthrough therapy highlight its potential to revolutionize the treatment landscape in the field of biomedicine.