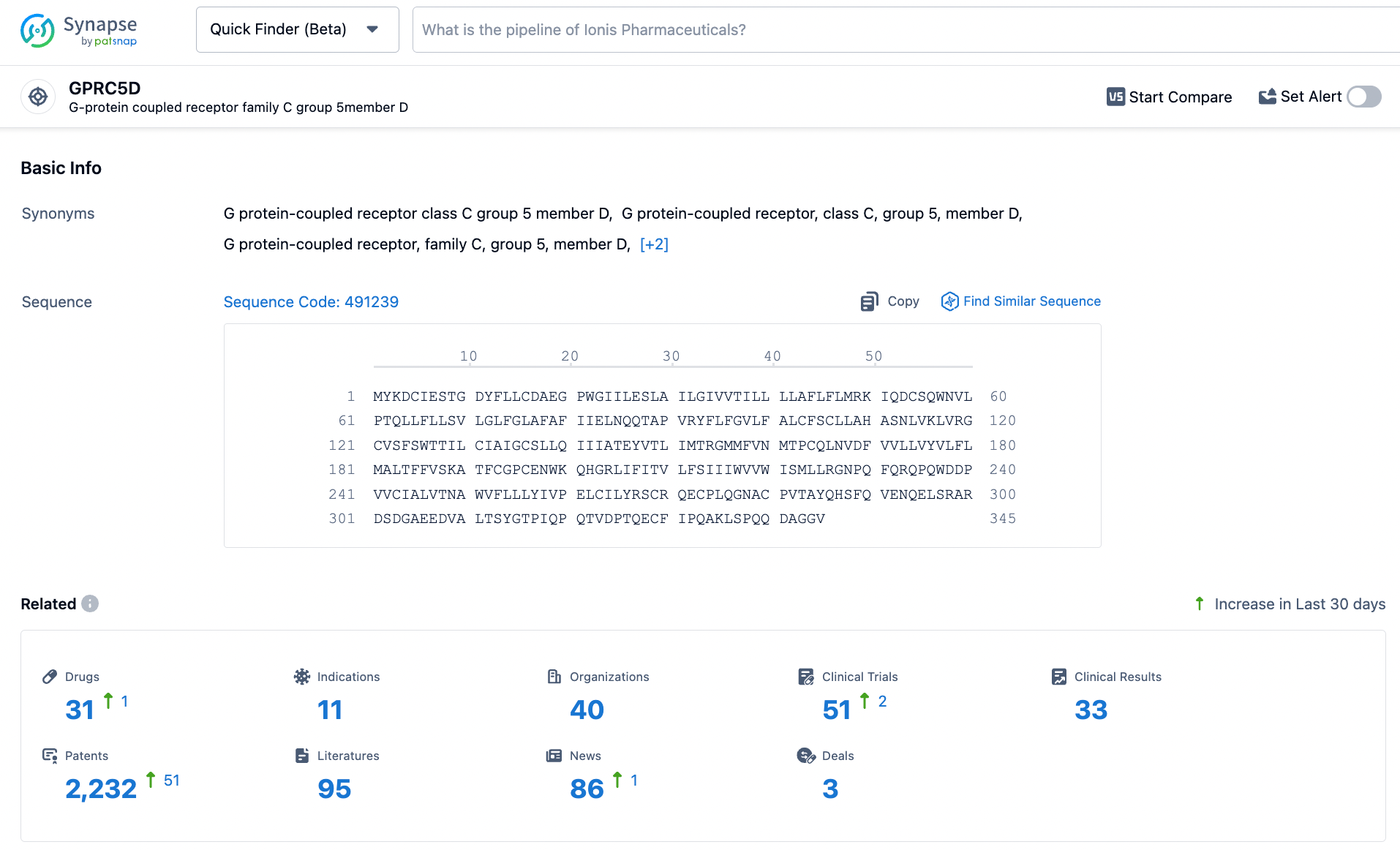
Targeted GPRC5D CAR-T Product: OriCAR-017
In January, Oricell Therapeutics announced that the U.S. FDA officially approved the Investigational New Drug (IND) application for OriCAR-017 for the treatment of patients with relapsed/refractory multiple myeloma (R/R MM).
It is reported that OriCAR-017 is a chimeric antigen receptor T cell therapy (CAR-T) targeting GPRC5D, developed by Oricell Therapeutics using its proprietary technology platform. The design and development of the product integrate Oricell's proprietary Ori®Ab antibody platform, Ori®CAR structural platform, and the company's proprietary CMC (Chemistry, Manufacturing, and Controls) technologies to achieve optimal conjugation, outstanding sustainability, and anti-tumor efficacy of the regenerated CAR-T cells. The IND enables Oricell to immediately initiate the clinical development of OriCAR-017 in the United States.
Prior to obtaining the FDA IND approval, OriCAR-017 had received NMPA IND approval in 2023, and clinical results from the researcher-initiated trial (POLARIS study) were published at the 2022 ASCO, the 2022 EHA, and in The Lancet Haematology journal.

According to an article published in the February 2023 issue of The Lancet Haematology, a team from Origene Biosciences evaluated the activity and safety of GPRC5D-targeted CAR-T cells in patients with relapsed or refractory multiple myeloma.
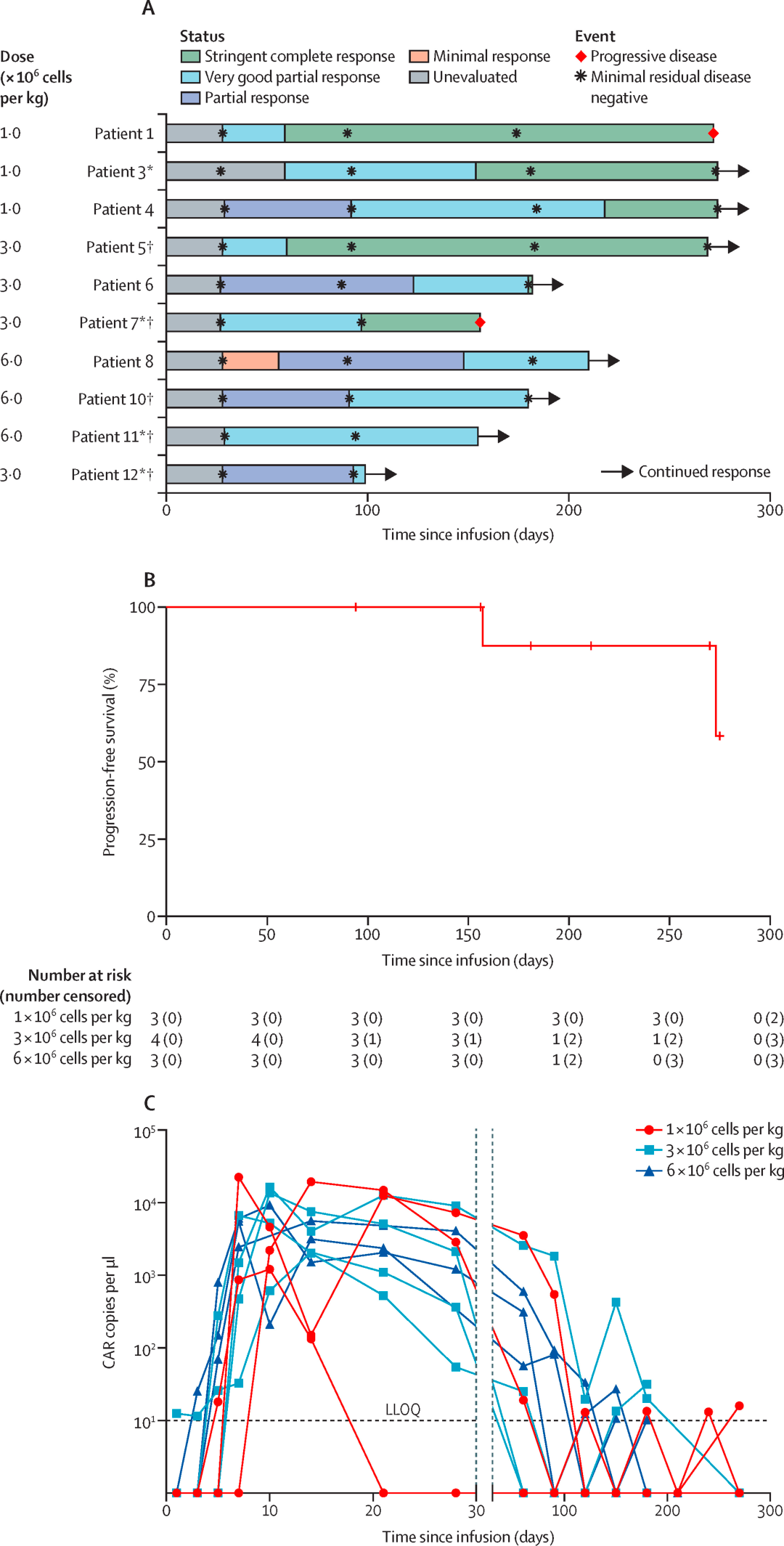
The study was based on a single-center, single-arm, phase 1 clinical trial named POLARIS. Eligible patients were aged 18-75 years with a diagnosis of relapsed or refractory multiple myeloma, had an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, and expressed more than 20% GPRC5D in bone marrow plasma cells or were immunohistochemically positive for GPRC5D, having previously received at least three lines of therapy including proteasome inhibitors, immunomodulatory drugs, and chemotherapy.
During the dose-escalation phase, patients were consecutively assigned to receive a single intravenous injection of OriCAR-017 at doses of 1×10^6 CAR-T cells per kilogram, 3×10^6 CAR-T cells per kilogram, or 6×10^6 CAR-T cells per kilogram. The primary endpoints were safety, maximum tolerated dose, and recommended dose for phase II.
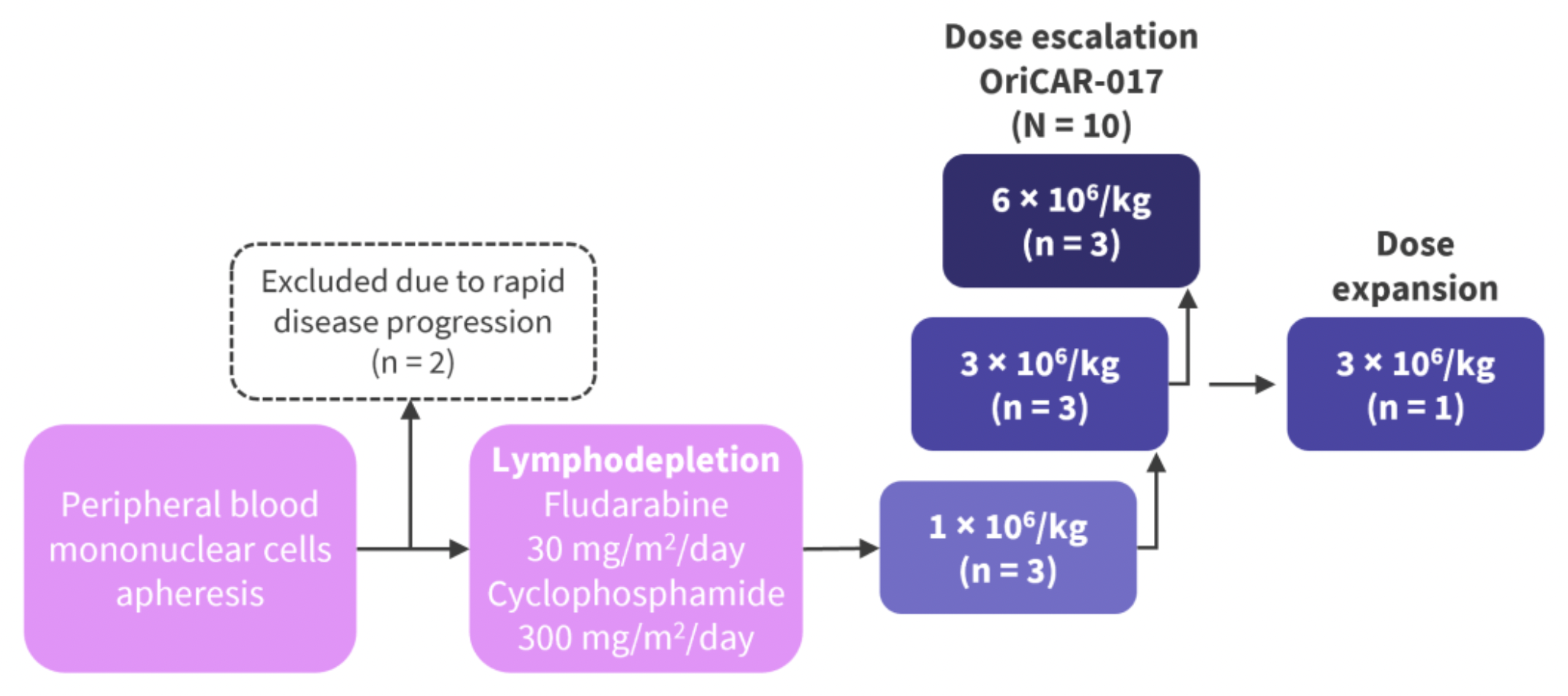
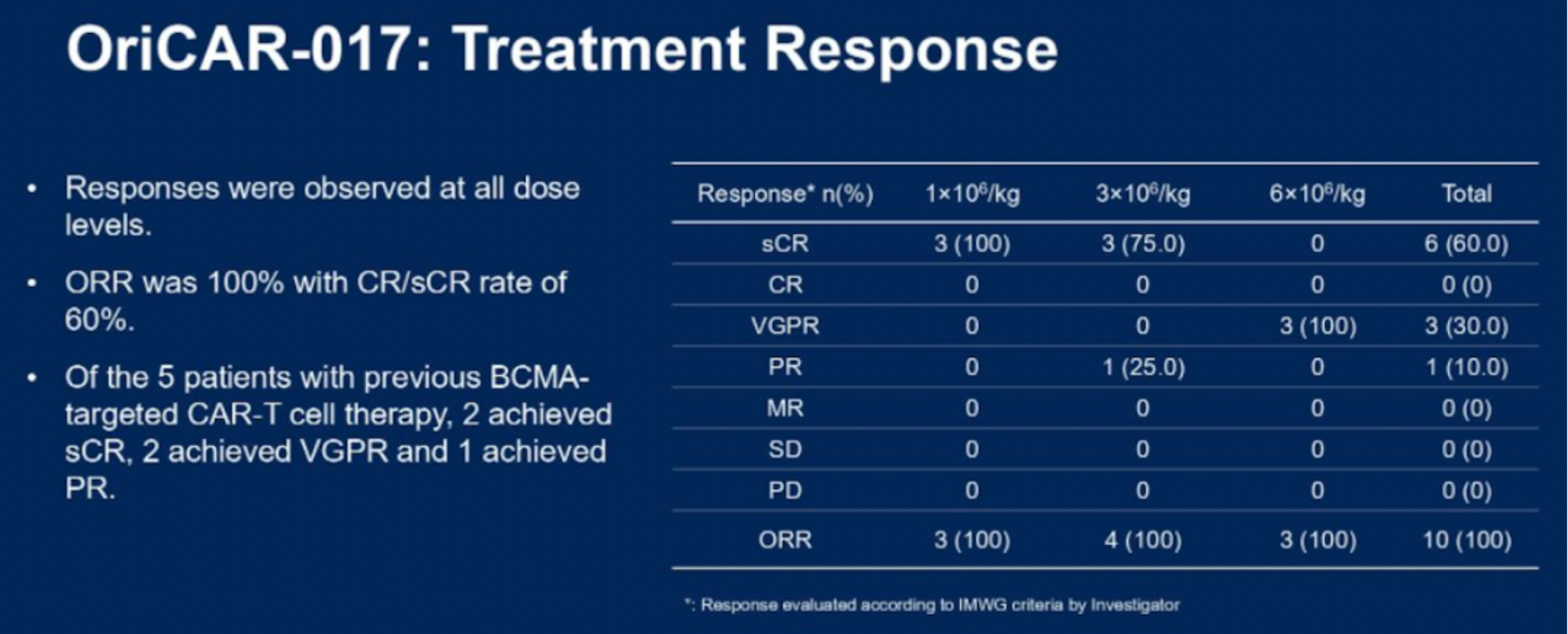
The data indicates that, according to the IMWG criteria, all 10 patients with relapsed/refractory multiple myeloma (R/R MM) responded to the treatment, with an overall response rate (ORR) of 100% and a stringent complete response rate of 80%. The therapy was well tolerated, with no incidences of immune effector cell-associated neurotoxicity syndrome (ICANS), no cerebellar disturbances, no delayed infections, and only grade 1/2 cytokine release syndrome (CRS), which resolved rapidly.
About Relapsed or Refractory Multiple Myeloma
Multiple Myeloma (MM) is a malignant hematological tumor disease involving plasma cells. It is referred to as "multiple" myeloma because this cancer typically affects multiple parts of the body, such as the spine, skull, pelvis, and ribs. Normally, plasma cells produce antibodies to help fight infections. However, in multiple myeloma, abnormal plasma cells begin to proliferate uncontrollably and accumulate in the bone marrow, leading to a range of clinical symptoms including bone destruction, anemia, and kidney function issues.
Relapsed or Refractory Multiple Myeloma (RRMM) is a term used in the field of oncology to describe a specific stage of multiple myeloma. It refers to a situation where the disease has recurred after a period of improvement or where previous treatment attempts have failed to elicit an adequate response.
The pathogenesis of RRMM involves multiple factors and processes, including genetic mutations that lead to clonal proliferation and abnormal growth of plasma cells; abnormal immune system function that allows plasma cells to evade immune surveillance; and aberrant changes in the bone marrow microenvironment that promote the growth and survival of tumor cells.
About Janssen's Talquetamab
In August 2023, Johnson & Johnson announced that the U.S. FDA has approved TALVEY™ (talquetamab-tgvs) for the treatment of adults with relapsed or refractory multiple myeloma.
Talquetamab is a bispecific T cell engager antibody that simultaneously targets the CD3 receptor on T cells and GPRC5D. GPRC5D is a novel drug target, classified as a Class C G protein-coupled receptor, which is found on certain normal cells but overexpressed on multiple myeloma cells.
In 2017, the Johnson & Johnson team first filed a global patent (WO2018017786) for the development of the Talquetamab antibody, which mentions that the patent provides antibodies that specifically bind to GPRC5D. The provided antibodies can be used for the diagnosis, treatment, or monitoring of progression, regression, or stability of cancers that express GPRC5D; to determine whether patients should receive cancer treatment; or to identify subjects with GPRC5D-expressing cancers, thereby assessing their eligibility for GPRC5D-specific anticancer therapy.
Regarding the discovery of antibodies related to GPRC5D x CD3, the Johnson & Johnson team has had a total of 8 related patent applications approved, and has conducted multiple clinical studies around MM (multiple myeloma) and RRMM (relapsed/refractory multiple myeloma).
In December 2022, the Johnson & Johnson team published for the first time in the New England Journal of Medicine an article on the Phase 1 clinical study results of Talquetamab, a T-cell redirecting bispecific antibody for GPRC5D, for the treatment of multiple myeloma.

The results showed that 232 patients were treated with talquetamab (102 by intravenous injection and 130 by subcutaneous injection). The median follow-up times were 11.7 months for patients who received a 405 microgram dose and 4.2 months for those who received an 800 microgram dose, with response rates of 70% and 64%, respectively. The median duration of response was 10.2 months and 7.8 months, respectively.
Common adverse reactions for the two subcutaneous injection doses (405 micrograms per kilogram weekly and 800 micrograms per kilogram every other week) were cytokine release syndrome (77% and 80% of patients, respectively), skin-related events (67% and 70%), and oral mucositis (63% and 57%); apart from one instance of cytokine release syndrome, all others were grade 1 or 2.
According to information from the official Johnson & Johnson website, the submission to the FDA for approval included the latest clinical trial data. In the Phase 2 MonumenTAL-1 study of talquetamab, which included 187 patients who had previously received at least four lines of therapy and had not previously received T cell redirecting therapy, the results demonstrated a meaningful overall response rate (ORR). At a subcutaneous (SC) dose of 0.8 mg/kg every two weeks, 73.6% of the patients achieved an ORR. During the median follow-up of nearly 6 months (0 to 9.5 months), 58% of the patients achieved a very good partial response (VGPR) or better, with 33% achieving a complete response (CR) or better.
Under a weekly SC dose of 0.4 mg/kg, 73.0% of patients achieved an ORR (Objective Response Rate). During a median follow-up of nearly 14 months after the first response (ranging from 0.8 to 15.4 months), 57% of patients achieved a VGPR (Very Good Partial Response) or better, with 35% achieving CR (Complete Response) or better.
Notably, the safety profile of TALVEY™ includes a “boxed warning” for cytokine release syndrome and neurotoxicity. The “Warnings and Precautions” section includes oral toxicity, weight loss, infections, cytopenias, dermatologic toxicity, hepatotoxicity, and embryofetal toxicity. The most common adverse reactions (≥20%) include fever, CRS (Cytokine Release Syndrome), dyspepsia, nail disorder, musculoskeletal pain, skin disorder, rash, fatigue, weight loss, dry mouth, xerosis, dysphagia, upper respiratory infection, diarrhea, hypotension, and headache. The most common Grade 3 or 4 laboratory abnormalities (≥30%) were lymphopenia, neutropenia, leukopenia, and anemia.
About GPRC5D
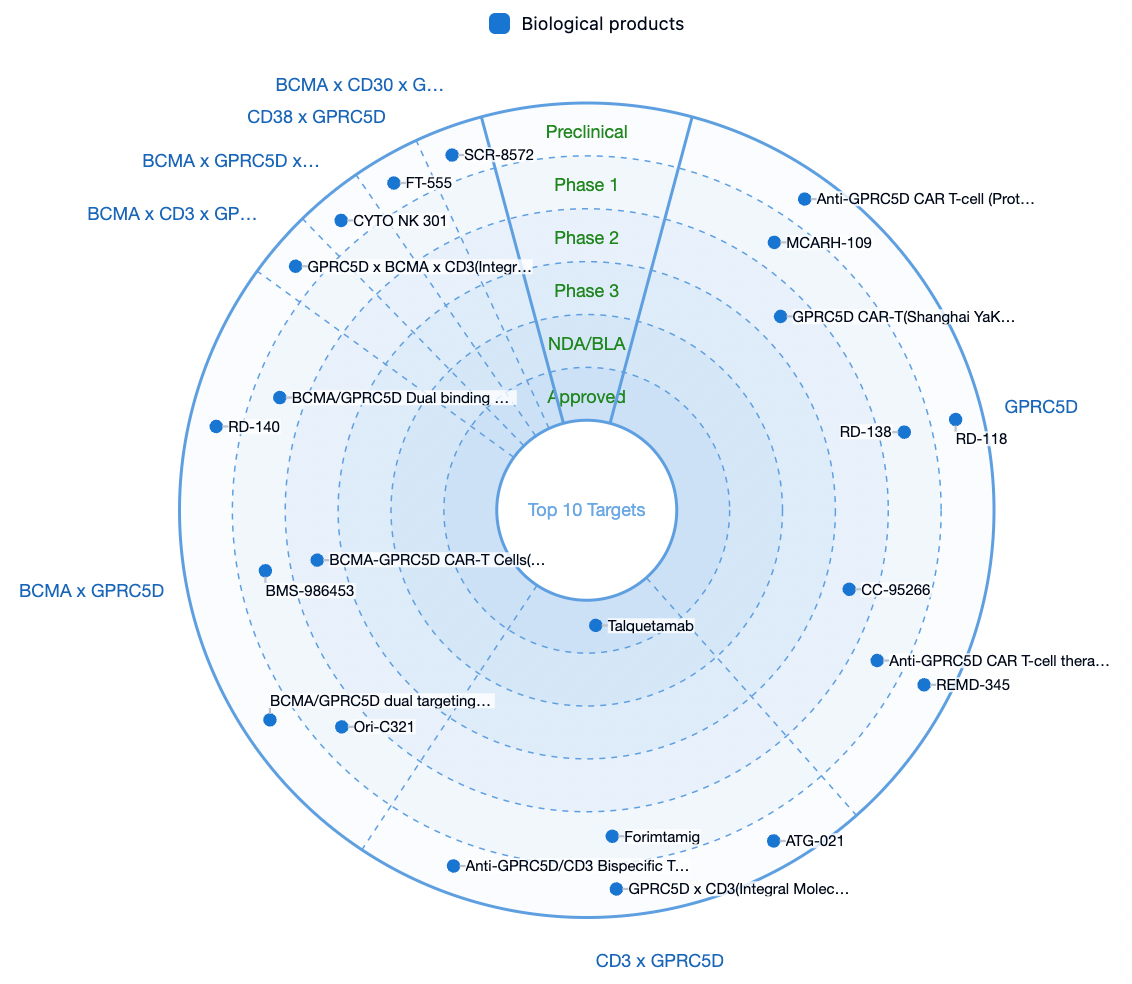
According to statistics from the Synapse database, there are a total of 31 drug pipelines worldwide targeting GPRC5D, among which only Talquetamab by Johnson & Johnson has been approved for marketing.
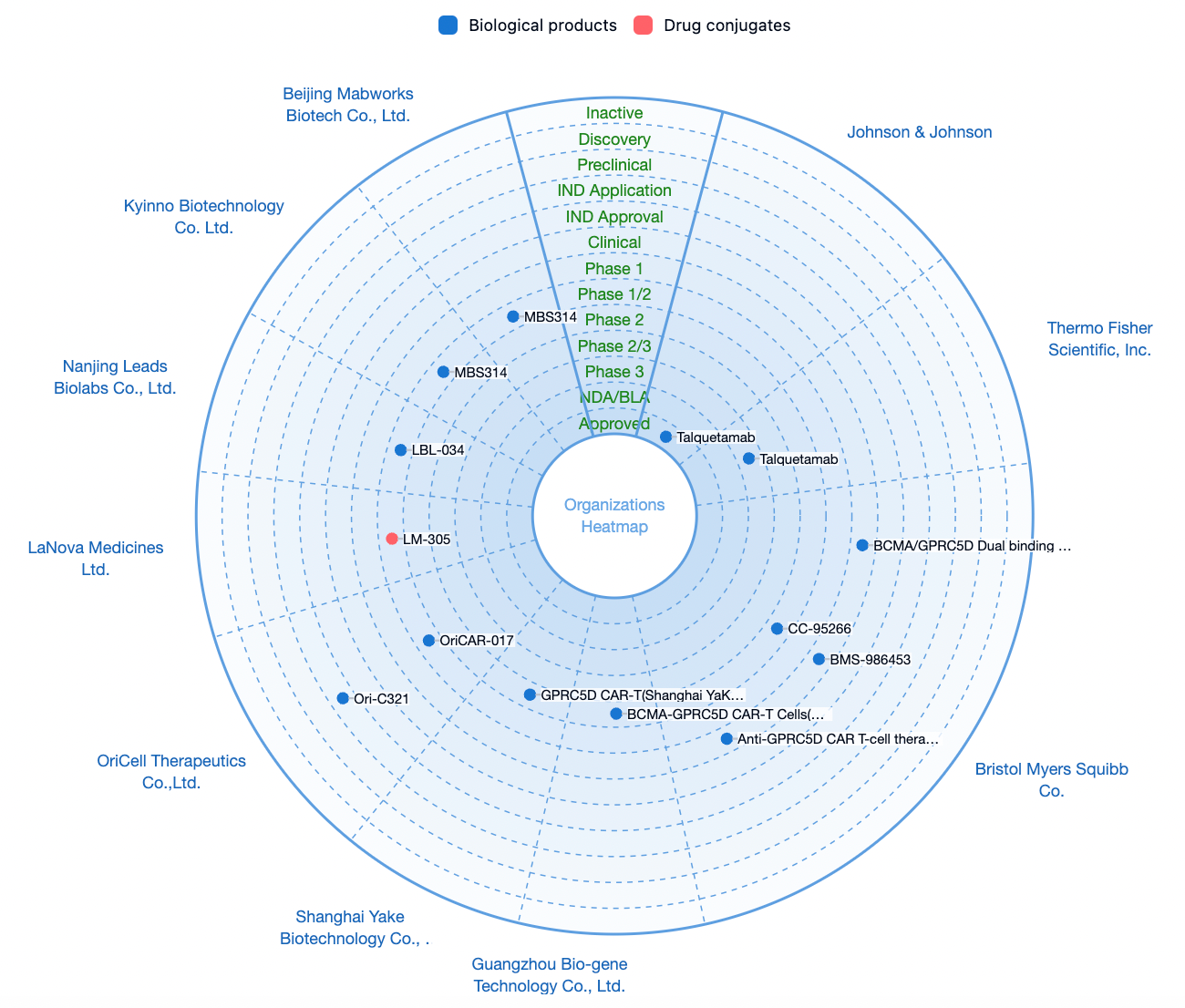
In addition to the independent development of biopharmaceuticals targeting GPRC5D, several pharmaceutical companies are focusing on the combination of GPRC5D with multiple targets, including CD3, BCMA, CD38, CD30, and others.
As an emerging potential target, GPRC5D is expected to see an increasing number of pharmaceutical companies entering the field of oncology as Johnson & Johnson's Talquetamab successfully launches.